
Blog
March 10, 2021
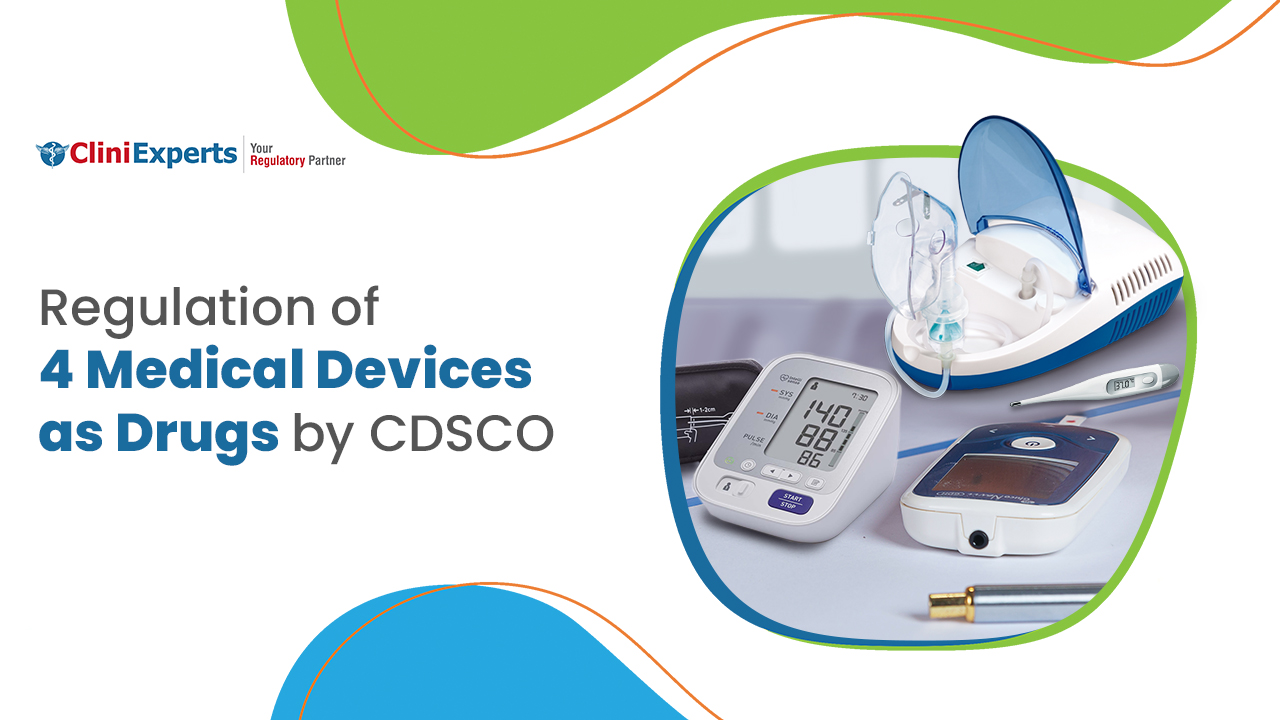
CDSCO has notified four medical devices, i.e., nebulizer, blood pressure monitoring devices, digital thermometer, and glucometer as drugs. The importers and manufacturers of these 4 medical devices need to take import and...

Blog
December 3, 2020

Indian pharma market is flooded with irrational Fixed Dose Combination (FDCs) and is an important topic for critical and scientific analysis. The country needs some regulatory laws for FDCs after analysing whether...

Blog
October 26, 2020

Coronavirus still poses a potential threat to the global population, but no vaccines protect the body against the virus, causing COVID-19. The coronavirus spreads quickly, and majority of the world's population is...

Blog
September 10, 2020

After the outbreak of COVID-19, the World Health Organization (WHO) has said that hand hygiene is important to prevent the virus's spread. Hand hygiene guidelines of WHO recommends the use of an...

Blog
August 20, 2020

CDSCO Extends Timeline For Import Of Drugs With Residual Shelf Life Less Than 60 Percent Till Oct 31
COVID-19 is disrupting global distribution on a scale unseen in recent times, creating extreme challenges for the supply chain of imported drugs and healthcare products. Increased border controls and customs regulations resulting...

Blog
June 12, 2020

As COVID-19 spreads across the globe, scientists are constantly trying to make effective and efficient drugs and vaccines and fast-track the development of diagnostic tools with better outcomes. The present situation demands research...

Blog
June 12, 2020

Manufacturing Licenses For New API (Active Pharmaceutical Ingredients) And Formulations There is a constant demand from customers and global competitive pressure to produce active pharmaceutical ingredients (API) on the pharmaceutical manufacturers in...

Blog
April 13, 2020

The Central Drugs Standard Control Organization (CDSCO) acknowledges the potential impact of COVID-19 pandemic on the healthcare system and broader society, and the impact it may have on clinical trials and subjects....






