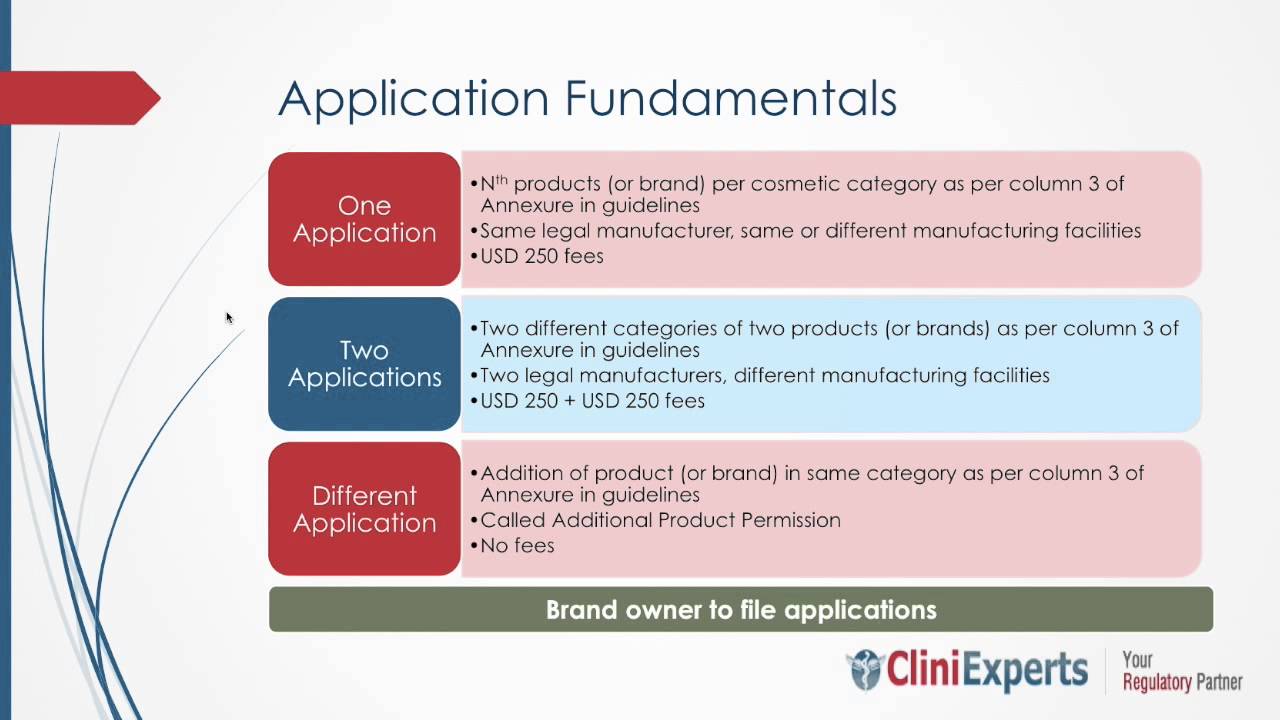
Complete Procedure to Meet the CDSCO License Mandate of Class C & D Non-Notified Medical Devices
Medical Device
October 25, 2023
Overview of the CDSCO License Mandate The Central Drugs Standard Control Organisation (CDSCO) is the regulatory body that manages medical device registration in India under the Ministry of Health & Family Welfare. The main aim of CDSCO is to ensure that medical devices are...
Read More


Blog
April 25, 2024

What is the 2006 FSSAI Act? According to the Food Safety and Standards Act of 2006, "An Act to consolidate the food-related laws, to establish the Food Safety and Standards Authority of...

Regulatory Update
April 10, 2024

Regulatory Update
March 8, 2024

Regulatory Update
February 21, 2024

Blog
February 14, 2024

Introduction In accordance with the 1940 Drugs and Cosmetics Act, the license is granted by the relevant State Drug License Authority. The Drug and Cosmetics Act of 1940 combines all the drug-related...

Blog
February 14, 2024

Introduction The primary concern of the Government is to ensure that every person has equal and fair access to drugs and medicines with affordable prices. The primary step in beginning drug production...

Regulatory Update
February 2, 2024
End-to-End Regulatory Solutions for Domestic and International Markets
ENQUIRE NOW
KNOW MORE, LEARN MORE, ENGAGE MORE.
“Video-Only” Resource For Ease Of Understanding