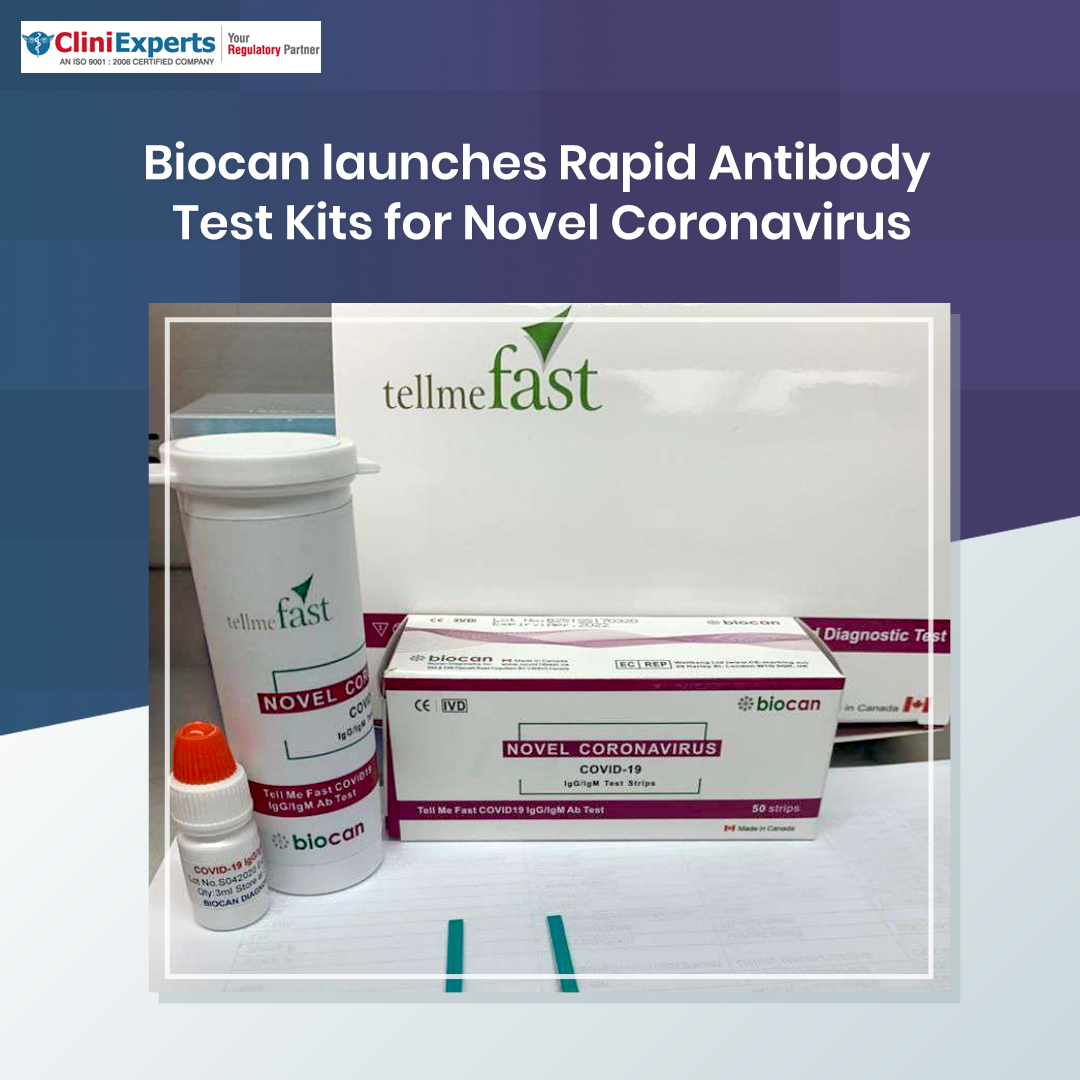
Biocan Launches Rapid Antibody Test Kits For Novel Coronavirus (COVID-19)
Biocan Diagnostic Inc brings in India a rapid testing kit named Tell Me Fast. A rapid test for the qualitative detection and differentiation of novel coronavirus (COVID-19) IgG & IgM antibodies in human whole blood, serum, and plasma samples, approved by the Central Drugs Standard Control Organisation (CDSCO) and (United States Food and Drug Administration) USFDA. CliniExperts helped in the rapid approval of the testing kit Tell Me Fast developed by Biocan Diagnostics Inc in India
Read MoreCLINICAL TRIAL ON MEDICAL DEVICE
Decided by the Expert Committee, the procedures for Clinical Trial approval, accreditations of investigators, sites, Ethics Committee and other conditions would be similar to the clinical trials of New Drug/ Vaccines.
Read MoreAPPLICATION TO MARKET NEW CHEMICAL ENTITIES (NCEs)
The Expert Committee decided that if India participates in GCT for NCE's to be used for diseases prevalent in our population, approval should be sought from CDSCO for marketing these NCEs in India after approval for marketing in the innovator country or in regulated markets.
Read MoreAPPLICATION TO MARKET NEW CHEMICAL ENTITIES (NCEs)
The Expert Committee decided that if India participates in GCT for NCE's to be used for diseases prevalent in our population, approval should be sought from CDSCO for marketing these NCEs in India after approval for marketing in the innovator country or in regulated markets.
Read More
CliniExperts Facilitating Fast Track Approval of COVID-19 Diagnostic Kits in India
Delhi based CliniExperts, a company that specialises in regulatory approval of diagnostic kits, has helped four diagnostic companies recently in getting Indian regulatory approval of their COVID-19 diagnostic kits
Read MoreANCILLARY CARE TO THE CLINICAL TRIAL SUBJECTS
Decided by the Expert Committee that there should be provision for providing ancillary care to patients suffering from any other illness during the trial.
Read MoreNews
Self-Assessment/Audit of Unit for GMP/GLP Compliance
The Indian government has brought about some major changes with regards to the rules and regulations governing the manufacturing to enhance the quality of products used in the healthcare industry. As India being a major market for the healthcare-related products and its services, these modifications to the existing regulations are […]
News
New Medical Device Rules To Be Rolled Out In 2018
The Drugs and Cosmetics Act, 1940, has been governing drugs and medical devices since 1940 and has received multiple iterations each year. Owing to the significant changes that have occurred in the healthcare and pharmaceutical industry and constant requests from leaders in these industries, an overhaul was long overdue. In […]
News
Top Five Trends Changing the Game in Global Healthcare IT
stethoscope in two years, preferring to examine his patients with a handheld ultrasound unit to view a patient’s heart in real-time. In 2012 alone, physicians in the U.S. are expected to increase their use of smartphones and tablets by 81 per cent.
