End-to-End Regulatory Solutions for Domestic and International Markets
We are a regulatory solutions provider serving the Drugs, Medical Devices, Biological, IVDs, Neutraceuticals, Food, and Cosmetic industries. We are committed to simplify, familiarize, and support our clients through every step of the regulatory journey.
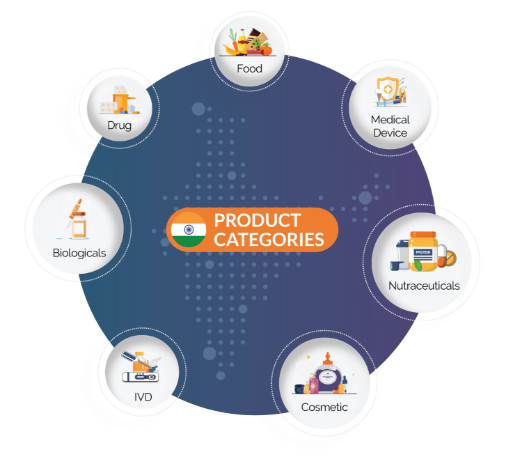

CliniExperts – your go-to Regulatory Solutions provider in India!
When a company enters a new market, it needs an expert who understands the complexities of the regulatory framework and can service all the mandates during the pre and after-market lifecycles from start to finish. Moreover, it should help integrate the company’s market strategies in the minimum possible time.
With hands-on experience of over twelve years, we bring value to the table, offering powerful insights and analysis across industry sectors, thorough uncompromising ethics, and unmatched quality.
Our standalone service features:
- To understand the Indian Industry and Regulatory landscape – the scenario, compliances and procedures.
- To identify the distribution network and associated challenges.
- To procure licenses and approvals.
- To study the Post Market Sustenance vis-à-vis the dynamic market and regulatory landscape.
Global outreach
With a talent pool of over 35+ experienced experts possessing up-to-date regulatory knowledge of India and several other countries, CliniExperts has a 2-way serve the overseas route. We serve both – Indian companies aspiring to enter the Indian market, and Indian companies having global expansion plans.
Our collaboration with vCare Denmark has deepened our overseas footprint, and we now serve the European region with this outreach.
Our Happy Clients
We work with the best companies from across the world. Our clients are well known globally for their high-quality products, services, post-sales service and customer care. They come with a commitment to be a part of the Indian Healthcare Sector.



































Team CliniExperts
Helps its Clients

We uphold the promise of walking with our clients every step of their journey in our vibrant Indian market.


Our Team
The CliniExperts Team is a blend of passion, professionalism, and perfection, comprising of competent and qualified professionals.

Surender Sharma 
- Assistant Manager, Administration
Surender is the Administrative expert at CliniExperts. He is always on his toes to ensure CliniExperts works seamlessly at all times. Surender often is the person who remembers or can locate information from our records. He comes with over 15 years of industry experience. He has proven himself to be a good manager.
Surender spends his spare time honing his skills as a singer and dancer.

Yusra Usman 
Manager - Regulatory Affairs
Yusra Usman comes with industry experience of over 10 years. She is the Food and Legal Metrology expert at CliniExperts and effectively handles the Regulatory Affairs for the same. She is process-oriented, and believes in following methods for even the smallest of concerns and ends up finding very quick solutions.
Yusra is keen on music and reading in her spare time.

Shipra Bisht 
Senior Manager – Business Development
Shipra with her over 11 years of industry experience adds value to the client relations at CliniExperts. She has diligently been part of the teams that worked with many new clients. She has always shown her sharp business acumen and organized manner of working.
Shipra loves music, painting and movies.

Ashwani Yadav
Senior Executive – Regulatory Affairs (Medical Devices)
Ashwani is one of our most efficient team members. He is a key member of the Regulatory Affairs team that manages the Medical Devices sector. Always a team person, he can be relied upon for great solutions to all concerns.
Ashwani loves to try new recipes and listen to music.
Vijay Maurya 
Senior Manager – Regulatory Affairs
Vijay Maurya, Senior Manager – Regulatory Affairs, has been a part of the Health Sector for over 12 years with a particularly deep understanding of the Drugs, Biologicals and Cosmetics. Vijay is known to be a very humble person.
He is an avid sportsperson.

Rajiv Kanwal
Senior Executive - Business Development
Rajiv Kanwal, Senior Executive - Business Development, has been a new addition to cliniExperts. He is responsible for Business Development.

Sonia Rana
Assistant Manager- Finance, brings 11 years of experience.
She is capable of streamlining processes by using her methodical approach. Sonia excels at math, and her compassionate and composed demeanor endear her to those who surround her.
At The Helm

Dr Ashwini Kumar is India’s leading clinical research expert. He is committed to advancing the Health Sector and taking good health to every citizen of the world. An alumnus of the prestigious AIIMS, he led many research projects for Pharmaceutical giants during his stint there. His commitment to medical research and penchant for ethics and perfection therein is well known.
His career in the Pharmaceutical Sector gave him unmatched insight into regulatory affairs, quality, training, and regulatory approval processes across the world.
His desire to make it possible for the Indian populace to have access to the best in the medical sector was the impetus behind his dream project ‘CliniExperts’. Established in 2009, it has since proven to be ‘The Go-To Company’ for navigating the regulatory space in India.
Ashwini is the force behind CliniExperts becoming the leading Regulatory Solutions provider in India and globally.
Dr Ashwini’s key role in saving lives during the Covid-19 pandemic:
When the pandemic struck India, Ashwini’s compassionate and concerted efforts made it possible to get fast-track approvals of Covid-19 testing kits. This was done in record time, which helped to save thousands of lives.

Rashmi Verma, the Chief Operating Officer at CliniExperts, is a tech whiz, Big Data Analyst & Software Developer. An alumnus of HBTI Kanpur, she has earned a formidable reputation as an Information Technology Expert.
The CliniExperts clients all know Rashmi as the go-to person to approach, for any help or assistance. Her key contributions at CliniExperts are, the Cosmoally App and the Food Safety Mantra blog. Looking at the dynamic regulatory scene in India, she has designed the software to track live changes in the Indian regulatory framework.
Rashmi is passionate about nurturing the health sector in India. She is an able leader and has spearheaded many projects helping companies to set up operations in India.
She was a key member of the Covid-19 Task Force at CliniExperts that ensured regulatory approvals for companies supplying Covid Testing Kits.
She wears multiple hats at CliniExperts, handling the IT, Operations, Policy & Finance departments. She is a visionary entrepreneur in words and action.
Rashmi loves to read books in her spare time. She is a doting mother to a very active four-year-old, who of course keeps her on her toes.
CliniExperts Promise
Quality at any cost, excellence at any price – This is not just a slogan, but a belief system that we live by, every moment of our corporate existence. Our zero-tolerance policy creates a zero-defect regulatory ecosystem, increasing the ease of doing business for our clients. Our formidable talent pool, knowledge base, and up-to-the-minute information about regulatory updates adds to this by providing the latest know-how to our clients.
The CliniExperts promise of excellence is the secret behind its successful implementation of over 1000 brand registrations, 500 product registrations, and 300 approvals obtained so far. The journey towards excellence continues unhindered, and our promise of quality at any cost, excellence at any price grows stronger with each passing year.
CliniExperts has Assisted
Covid-19 pandemic hit the world, we at CliniExperts successfully registered Covid-19 detection kits in the shortest possible time. We continue to work for humanity and our country.
Corporate milestones
CliniExperts Services Pvt Ltd is the culmination of the vision of Dr Ashwini Kumar, MD (AIIMS). He envisioned being a partner to global entrants to the Indian market.
He decided to be ‘The Regulatory Solutions Provider for India’ to companies from the health sector in Medical Devices / diagnostic kits, Pharmaceuticals, Food / Nutraceuticals, Cosmetics, Biotech and others. He dreamt of empowering the Indian Health Sector and the Indian populace. And, it was in 2009 that his dream became a reality.
20202020 – CliniExperts played a key role in obtaining fast-track approvals for Covid-19 testing kits during the pandemic. This helped in timely detection of the virus and saving thousands of lives. 
20182018 – CliniExperts set up its Singapore operations with a dedicated research center. Over 300 approvals obtained. 
2015ISO 9001: 2008 Certification; over 200 clients; Entrepreneur of the Year Award for Dr Ashwini Kumar, CEO 
2013By 2013, Cliniexperts served 75 companies to get registered in India 
|

20192019 – A landmark year. Cosmoally App was launched, FoodSafetyMantra, A Blog on Food Regulatory Updates went live, Over 350 approvals obtained. The company breached the 500-clients threshold. 
20172017- CliniExperts entered the India Market Division. We participated in the German Medical Device Trade Mission and received a record 280 approvals! 
20142014 CIO Reviews featured us as one of 20 most promising Healthcare & Life Sciences Software companies in India. |

20202020 – CliniExperts played a key role in obtaining fast-track approvals for Covid-19 testing kits during the pandemic. This helped in timely detection of the virus and saving thousands of lives. 
20192019 – A landmark year. Cosmoally App was launched, FoodSafetyMantra, A Blog on Food Regulatory Updates went live, Over 350 approvals obtained. The company breached the 500-clients threshold. 
20182018 – CliniExperts set up its Singapore operations with a dedicated research center. Over 300 approvals obtained. 
20172017- CliniExperts entered the India Market Division. We participated in the German Medical Device Trade Mission and received a record 280 approvals! 
2015ISO 9001: 2008 Certification; over 200 clients; Entrepreneur of the Year Award for Dr Ashwini Kumar, CEO 
20142014 CIO Reviews featured us as one of 20 most promising Healthcare & Life Sciences Software companies in India. 
2013By 2013, Cliniexperts served 75 companies to get registered in India |
CliniExperts in Media
CliniExperts – Most Promising Medical Device & Pharmaceutical Regulatory Consultant From Asia
CliniExperts proudly shares that Business Outlook Magazine has featured us as a top 10 Regulatory and Pharma consultant in Asia on their cover story in the September edition.
Healthcare Budget 2023-24 gets its deserved due Insights from CliniExperts CEO
Budget 2023-24 is both path-breaking and path-setting for the industry. CliniExperts CEO Dr Ashwini Kumar decodes here the far-reaching implications of budgetary announcements for the healthcare sector.
CliniExperts and vCare Denmark come together for inclusive and holistic Regulatory Solutions
We have entered into a strategic collaboration with vCare Denmark, a holistic and inclusive healthcare company with a wide network in the EU. This collaboration will give us opportunities to serve the European companies aspiring for India Entry.
Testimonials
The Brand That Promises To Turn, Your Business Around!
Atomy India Pvt ltd
We have been associated with CliniExperts for about a year now for our food products related services for FSSAI compliances and licenses from CliniExperts and are completely satisfied with their performance. Quick turnaround time in responding to queries, meticulous planning in completing the task, alongside the extensive knowledge of the regulations of the entire team is highly appreciable and marks the professionalism and quality driven ethics of the organization. We highly recommend their services as we believe it is best in the industry so far.

Rajeev Rattava
Finance Manager
Morepen Laboratories Ltd.
The team at CliniExperts is proficient in the subject matter and provide prompt services . We are satisfied with our engagement and would recommend their services.

Anubhav Suri
Business Head
Proficon Medisol Pvt. Ltd.
We have been professionally associated with Cliniexperts for more than 5 years now. They have smoothly sailed us all these years in different projects & this has been possible because of the knowledge & dedication cliniexpert team has in their portfolio. All the best Cliniexpert. Keep going.

Ajitesh Trehan
Director
Unity Enterprises
We appointed Cliniexperts for our Cosmetic registration project for one of our critical products for which we received the registration certificate much before the timelines committed by the company. The entire project was handled efficiently with sheer professionalism and in our comfort zone . We are very happy with the overall working experience with CliniExperts and will highly recommend their services to everyone.

Faiyaz
Founder, Unity Enterprises

