Wireless Medical Devices
CliniExperts manage them expertly FSSAI regulatory affairs
Full-funnel services Import, market, operate, and maintain with confidence
Food business in India is becoming increasingly lean and mean. With recent reforms kicking in, the sector is on its way to achieving global regulatory benchmarks. Rather than risking in-house attempts, we entrusted the function to CliniExperts. We are glad we did. No pain, only gain!
 |
FosCos FSSAI Food License & Registration in India |
 |
Non Specified Product / Ingredient Approval in India – Form I & II |
 |
Fssai Compliance (Food Regulation) - Food Safety & Standards -CliniExperts |
 |
FSSAI Authorized Agent Support For Food |
 |
FSSAI Food Claim Approval in India |
 |
Approval of Non Specified Ingredients/ Products - For Food Supplements & Nutraceuticals |
 |
FSSAI License for Food Supplements and Nutraceuticals in India – Form B & Form C |
 |
FSSAI Advertisement & Claims Approval - For Food Supplements & Nutraceuticals in India |
 |
FSSAI Compliance For Food Supplements & Nutraceuticals |
Partner with Cliniexperts, and get the advantage of experience, expertise, efficiency, and integrity.
Food safety – Food for thought
With over 2,000main food palettes, India is a food continent.With such a vast and diverse array of choices, food safety becomes a prime concern for one and all. The Ministry of Health and Family Welfare has established Food Safety and Standards Authority of India (FSSAI) as the parent regulatory body, with outreach in states and union territories through regional offices. The governing law is the Food Safety and Standards Act, 2006.
FSSAI has introduced sweeping reforms in this act through the Food Safety and Standards (Amendment) Bill 2020, which has enforced an enhanced regulatory framework across product, packaging, and raw material categories. These reforms give more teeth to the law and empower it to further safeguard citizens’ food safety and security, while enhancing the punitive mechanism against erring parties.
As the laws get more stringent, so do the regulatory mandates. In such a reformed ecosystem, manufacturers and marketers need an agile and competent regulatory partner who can deliver cutting-edge solutions from start to finish. That’s why, CliniExperts – The one-stop regulatory service provider for the entire food sector.
As the laws get more stringent, so do the regulatory mandates. In such a reformed ecosystem, manufacturers and marketers need an agile and competent regulatory partner who can deliver cutting-edge solutions from start to finish. That’s why, CliniExperts – The one-stop regulatory service provider for the entire food sector.
About FSSAI?
It was established in 2008 with the prime objective of consolidating the hitherto decentralised rules and laws related to food safety, and providing a centralised, single-window governing and regulatory body. Thus, FSSAI acts as the main enforcer of the Food Safety and Standards Act, 2006 and its amends. It lays down “science based” standards for food items across categories and regulates their manufacture, storage, distribution, sale and import.

Who Can Apply?
About Foscos
- Launched in 2020, the Food Safety Compliance System or FosCoS is the second-generation licensing and registration system for food manufacturers and allied industries.Replacing the erstwhile Food Licensing and Registration System (FLRS), FosCoS offers a centralised pan-India virtual platform that facilitates all regulatory functions online. This techno-savvy solution has enabled digital transformation of the entire registration, licensing, and regulatory funnels of the food sector.
- The FoSCoS vision is to integrate all aspects and domains of food safety regulatory needs under one digital umbrella. Several functions, including Food Safety Compliance through Regular Inspection and Sampling (FoSCoRIS), Indian Food Laboratory Network (InFoLNet), Food Safety Training and Certification (FoSTaC), will ultimately integrate with FoSCoS, thus transforming it into a unified food regulatory system.
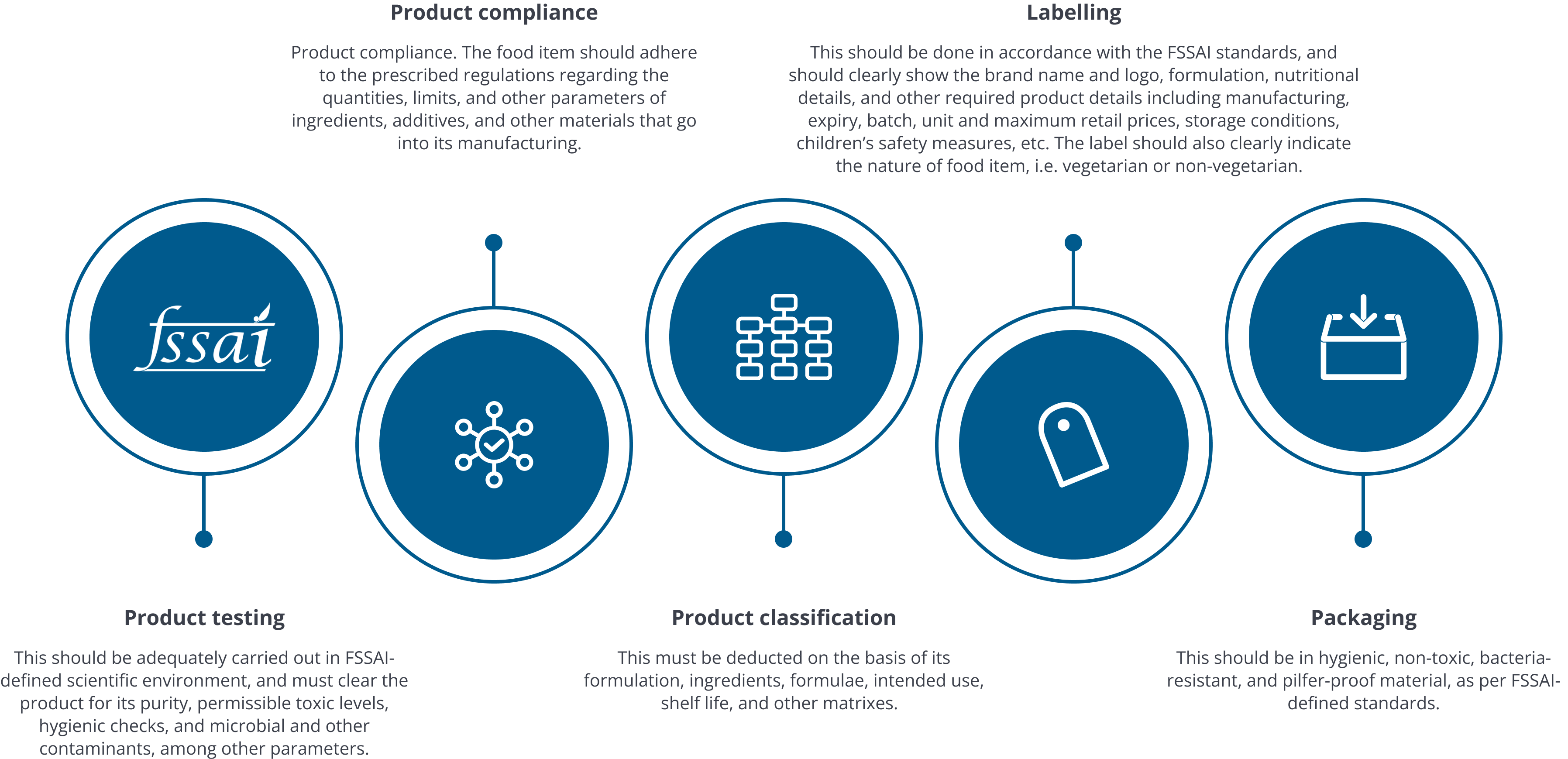
Registration and licensing
As per FSSAI standards, all petty merchants and traders, hawkers, and other sundry sellers/resellers of food products with annual turnover below RS. 12 Lakh are required to be registered with FSSAI. All other parties, including manufacturers, distributors, retailers, and large sales outlets are required to obtain an FSSAI license.
Why Choose CliniExperts?
The lengthy and cumbersome food regulatory process involves over 320 steps, and at each step, a high degree of factual accuracy and error-free reporting is required. Such a complex, detail-oriented, and knowledge-based regulatory function is best left to domain experts. Attempts at self-fulfilling this requirement may result in loss of valuable time and effort, and in cases of delay and penalties, loss of business and income. As an astute business person, would you rather not have a trusted regulatory partner handle your regulatory matters?
With over 13 years of hands-on experience and expertise, CliniExperts is your best bet for a long-term, mutually rewarding regulatory partnership. As a satisfied client articulates it:

 |
FosCos FSSAI Food License & Registration in India License is obtained in Form C INR 100 per year is the registration fee for taking the FSSAI license to begin any food business on any premises whereas INR 2000/ 3000/ 5000 per year are the fees prescribed for obtaining a State License for the food business activities and INR 7500 per year for obtaining Central License. |
 |
Non Specified Product / Ingredient Approval in India – Form I & II Non specifed food approval is obtained in Form II To obtain non-specified food approval by FSSAI, the applicant is required to pay INR 50,000 per application. |
 |
Manufacturer licenseFSSAI Compliance (Food Regulation) - Food Safety & Standards -CliniExperts FSSAI Food Product compliance is a procedure where the applied food product meets all the requirements in the form of directives, regulations, and standards stated by FSSAI. There are 320+ checkpoints to be kept in mind for obtaining food product compliance in the fastest timeframe with minimal hindrance. |
 |
FSSAI Authorized Agent Support For Food
|
 |
FSSAI Food Claim Approval in India
|
 |
Approval of Non Specified Ingredients/ Products - For Food Supplements & Nutraceuticals
|
 |
Approval of Non Specified Ingredients/ Products - For Food Supplements & Nutraceuticals
|
 |
FSSAI License for Food Supplements and Nutraceuticals in India – Form B & Form C
|
 |
FSSAI Advertisement & Claims Approval - For Food Supplements & Nutraceuticals in India
|
Testimonials
The Brand That Promises To Turn, Your Business Around!
Atomy India Pvt ltd
We have been associated with CliniExperts for about a year now for our food products related services for FSSAI compliances and licenses from CliniExperts and are completely satisfied with their performance. Quick turnaround time in responding to queries, meticulous planning in completing the task, alongside the extensive knowledge of the regulations of the entire team is highly appreciable and marks the professionalism and quality driven ethics of the organization. We highly recommend their services as we believe it is best in the industry so far.

Rajeev Rattava
Finance Manager
Morepen Laboratories Ltd.
The team at CliniExperts is proficient in the subject matter and provide prompt services . We are satisfied with our engagement and would recommend their services.

Anubhav Suri
Business Head
Proficon Medisol Pvt. Ltd.
We have been professionally associated with Cliniexperts for more than 5 years now. They have smoothly sailed us all these years in different projects & this has been possible because of the knowledge & dedication cliniexpert team has in their portfolio. All the best Cliniexpert. Keep going.

Ajitesh Trehan
Director
Unity Enterprises
We appointed Cliniexperts for our Cosmetic registration project for one of our critical products for which we received the registration certificate much before the timelines committed by the company. The entire project was handled efficiently with sheer professionalism and in our comfort zone . We are very happy with the overall working experience with CliniExperts and will highly recommend their services to everyone.

Faiyaz
Founder, Unity Enterprises
Frequently Asked Questions
What sales model you are offering?
Focus on your business and increase the reach in India market, as we are acting a neutral party with taking care of registration, import and seamless logistics support round the clock

What is the date of enforcement for the Food Safety and Standards (Labelling and Display) Regulation, 2020?
The timeline for mandatory compliance with labelling display regulation as per the Food Safety and Standards (labelling and Display) Regulation, 2020 has been extended to the 1st of July 2022.

What happens if the product is non-compliant?
There are provisions relating to offences and penalties specified in the Food Act if the product is non-compliant. Here is a list of a few of them: - A penalty of 5 lakhs on the manufacturer, seller, storage, distributor, and importer for sub-standard food. - A penalty of 3 lakhs on the manufacturer, seller, storage, distributor, and importer for misbranded food. - A penalty of 10 lakhs on the publisher or any person involved in publishing for a misleading advertisement describing false description, nature, or quality of substance.

Is the FSSAI License under Importer KoB sufficient to carry out further food Business activities of Imported Food Products?
No, FSSAI License under Importer KoB is only for importing food Items into the Country. Therefore, for carrying out further food business activities of that imported food products, as defined under clause 3(n) of the Food Safety and Standards Act, 2006, FBOs need to endorse all such relevant Kind of Business categories in the issued license by applying modification of license.

Do the importer and manufacturer require separate licenses for carrying different food businesses within the same premises?
No, the applicant must fill out all the details in one form for obtaining an FSSAI license for more than one food product.

What types of food products or ingredients are covered under these regulations?
The following food products or ingredients are covered under these regulations: - Novel food or novel food ingredients. - New additives or new processing aids (including enzymes). - Articles/papers of food products or ingredients that contain or are isolated from microorganisms, bacteria, yeast, fungi, or algae. - Other non-specified food.

What is a new additive as per the FSSAI?
A new additive can be defined as an ingredient that has not been included in any specific food category per the Food Safety and Standards Regulations (FSSR). The products listed under this category also include the additive assessed for safety by the Joint FAO/WHO Expert Committee on Food Additives (JECFA). The food additive is included in Codex and approved by other regulatory authorities.

How many claim statements can be addressed per application?
A maximum of three claim statements can be addressed in one application.

