FOOD
Exploring Functional Food Supplements: A Manufacturer's Handbook
Empowering Manufacturers with Insights and Strategies for Functional Food Supplement Production
CliniExperts: Your Path to Success Starts Here
Embark on your regulatory journey with confidence, knowing that our seasoned team is dedicated to providing a one-stop solution for your project from start to finish. With our extensive regulatory manpower support, we ensure that you have the expertise you need at every step of the way. From navigating complex food product scientific details to guiding you through critical regulatory documents, we're here to ensure compliance and success. Our team offers expert guidance on clinical trial objectives and parameters, along with support in selecting appropriate testing labs, so you can navigate the regulatory landscape with ease and efficiency. Trust us to be your partner in achieving regulatory excellence and bringing your products to market seamlessly.
FSSAI: Regulating Food Businesses for Safety and Compliance
The Food Safety and Standards Authority of India (FSSAI) operates as a statutory body under the Ministry of Health and Family Welfare, Government of India.
Its main responsibility involves overseeing the production, storage, distribution, sale, and import of food items, with a focus on ensuring their safety and quality. FSSAI sets science-based standards for food products, with the ultimate goal of providing safe and nutritious food for human consumption.
CliniExperts possesses the experience and expertise to seamlessly navigate the entire regulatory process with an impeccable track record for accuracy.

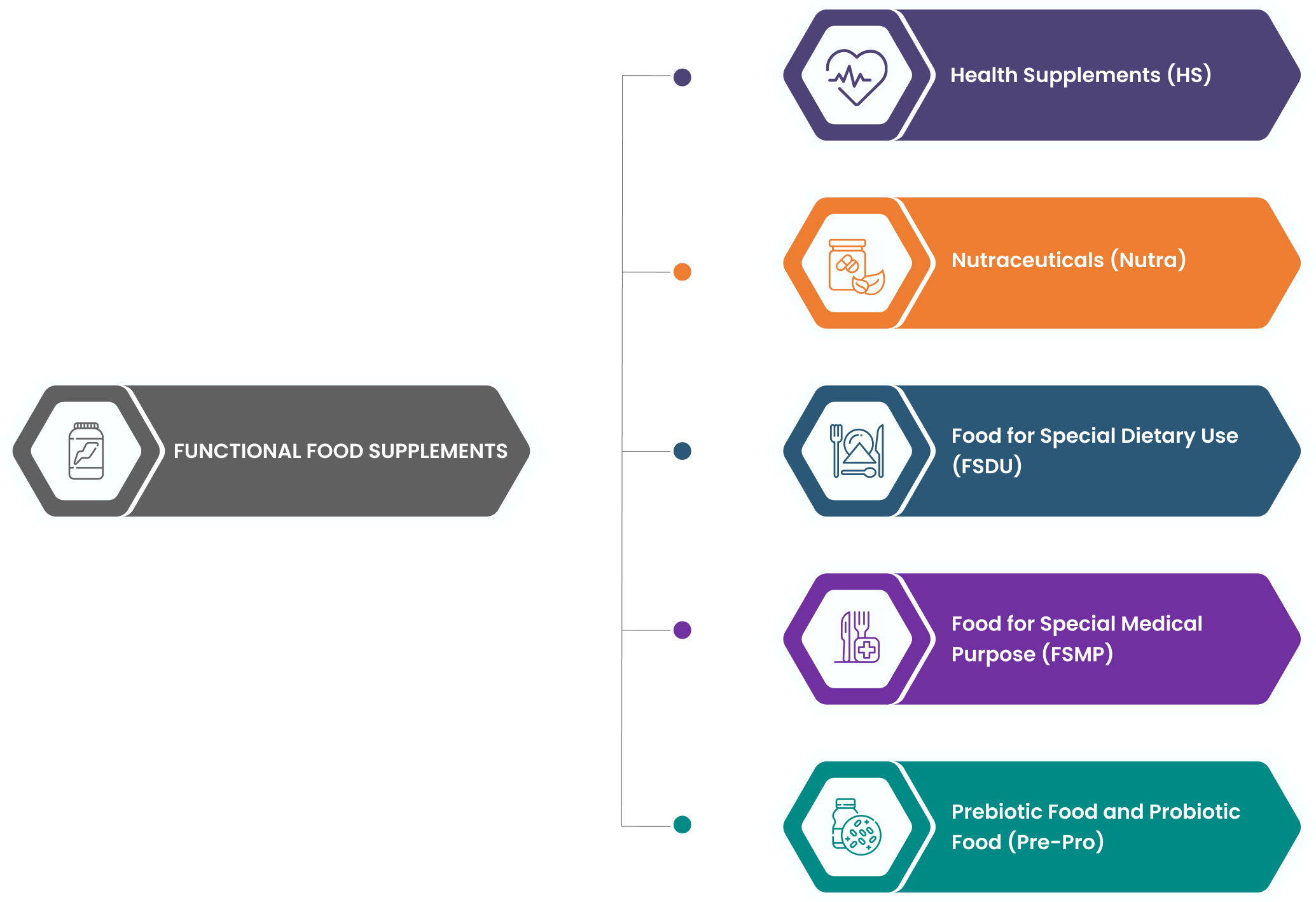
About Functional Food
- Functional foods are food ingredients that provide health benefits beyond basic nutrition.
- Certain types may include supplements or other added components aimed at enhancing health.
- These products are specially processed or formulated for specific nutritional or dietary purposes, distinguishing them from standard foods.
- They are meant to be consumed orally in specified quantities and duration and do not include products intended for intravenous use.
- Health Supplements ( HS)
- Nutraceuticals (Nutra)
- Food for Special Dietary Use (FSDU)
- Food for Special Medical Purpose (FSMP)
- Prebiotic Food and Probiotic Food (Pre-Pro)
Health Supplement (HS)
HS are a type of food containing concentrated nutrients such as proteins, minerals, vitamins, and amino acids, either individually or in combination. They are intended to supplement the regular diet by providing additional nutritional or physiological benefits.


Nutraceuticals (Nutra)
Nutraceuticals encompass extracts, isolates, and purified chemical compounds that offer physiological benefits and contribute to maintaining health.


Food for Special Dietary Use (FSDU)
FSDU are specially processed or formulated to meet specific dietary needs arising from certain physical or physiological conditions, diseases, or disorders. Their composition must notably differ from ordinary foods of similar nature, if such exist. Food supplements for the management of diseases or disorders, to be used under medical supervision, are typically categorized as Food for Special Medical Purposes (FSMP).


Food for Special Medical Purpose (FSMP)
These are foods designated for special medical purposes, formulated and presented for the dietary management of patients. They are used under medical supervision and are intended for patients with limited ability to consume, digest, absorb, or metabolize ordinary foods, or those with specific medically-determined nutritional needs. These foods are necessary when dietary modification or other specialized dietary products alone cannot meet the patient's nutritional requirements.


Prebiotic and Probiotic Food (Pre-Pro)
Prebiotic food refers to food containing additional ingredients that are non-living components providing health benefits by influencing gut microbiota.
Probiotic food contains live microorganisms beneficial to human health, which, when consumed in sufficient quantities either individually or as a blend of cultures, offer specific health benefits.

Why Choose CliniExperts?
Trust in CliniExperts's excellence to unlock the potential of functional food supplements manufacturing in India.
Contact our team to get the best support for regulatory services in India.


Ways CliniExperts Assists Clients
Clients primarily choose us for four key reasons:
Our steadily growing client roster and 100% retention rate speak volumes. When seeking a regulatory partner, clients prioritize market stature and size, and CliniExperts excels in these aspects.
Ethics, integrity, and excellence define our approach. We are a responsible and respected entity that navigates regulatory dynamics with pragmatism and ample resources.
Our teams, led by industry frontrunners, showcase unmatched talent and commitment to quality across all functions.
We pride ourselves on staying constantly updated with cutting-edge knowledge and information, ensuring that our applications are perfectly compliant in every aspect.
Together, these attributes often earn us the esteemed status of “Most Favored Service Provider."
Let's collaborate for a mutually rewarding and enduring partnership!
Form Names: NA
Food Product Compliance For Food Supplements
Ensuring food product compliance is crucial to guarantee that products manufactured meet FSSAI norms comprehensively. Compliance involves adhering to all standards and regulations to avoid penalties or legal repercussions for the Food Business Operator (FBO). Maintaining compliance not only mitigates risks but also fosters the establishment of a trustworthy brand.
Regulatory Body Requirement
FSSAI Food Product compliance ensures that products adhere to the essential requirements set forth by FSSAI through directives, standards, and regulations. It applies to manufacturers, marketers, sellers, and FBOs involved in storing and distributing food supplements and nutraceuticals. Compliance with FSSAI Product regulations is overseen by FSSAI and related bodies like Legal Metrology, where applicable. The service aims to verify that product formulations and labels align with FSSAI standards, involving an analysis of the formulation to ensure compliance with regulations.
Essential Tips
Here are some key tips for achieving FSSAI food compliance:
- Ensure accurate listing of all ingredients and additives.
- Adhere to the quality standards set by FSSAI for the product.
- Ensure compliance with the latest Food Safety and Standards (Labelling and Display) Regulations, 2020, for the product label.
- Consider reformulating the product, if necessary, to eliminate non-compliant ingredients or to enhance its value and potentially bypass the need for NSF approval.
- Step 5: After the review, the State Licensing Authority will grant the manufacturer permission and the manufacturing license in Form MD-5
Form Names: NA
FSSAI Advertisement Claim Approval For Food Supplements
FSSAI advertisement claim approval ensure that advertisements align with food labels, avoid deception, and provide clear information to consumers. Misleading claims can result in penalties, emphasizing the importance of claim approval for food businesses.
Regulatory Body Requirement
The Food Safety and Standards Authority of India (FSSAI) oversees Claims and Advertisements for Food Supplements and Nutraceuticals in India. Manufacturers of nutritional and health products must obtain prior approval before making any health or nutritional claims, or claims related to the reduction of disease risk, to ensure consumers are not misled by advertisements. The FSS (Claims and Advertisements) Regulations, 2018, aim to promote fairness in the claims and advertisements of food products.
How to Apply
To obtain FSSAI Advertisement & Claims Approval, the applicant should follow the below procedure:- Step 1: The applicant must check for the feasibility of claim approval associated with the ingredient/nutrient/product.
- Step 2: The applicant must prepare the Claim Support Dossier (CSD) in prescribed formats along with data suggested by FSSAI.
- Step 3: As a pre-requisite charge, the applicant must prepare a demand draft of Rs. 50,000.
- Step 4: The applicant must submit the duly filled application form, CSD, and demand draft to the FSSAI Office.
- Step 5: The maximum claim per application is three claims.
- Step 6: Finally, the applicant must wait until the application scrutiny/query/approval/rejection is done.
Form Names: Form B & C
FSSAI License For Food Supplements
The acquisition of an FSSAI license is a pivotal step for Food Business Operators (FBOs) in ensuring the safety, quality, and integrity of the food products they offer to consumers. This license serves as a hallmark of compliance with stringent food safety standards and regulations mandated by the FSSAI, thereby affirming that the food is safe for consumption and does not pose any adverse health consequences.
Regulatory Body Requirement
The Food Safety and Standards Authority of India (FSSAI) oversees food-related licensing in India. Manufacturers of functional food supplements are required to possess a valid FSSAI License. This license ensures that Food Business Operators (FBOs) are registered with FSSAI, facilitating compliance with regulations such as food safety audits, annual returns, product testing, and recalls. It serves as a mechanism for FBOs to ensure adherence to FSSAI licensing guidelines.
How to Apply
To obtain an FSSAI License, applicants must follow these steps:- Step 1: Visit the FoSCoS website for food licensing and registration.
- Step 2: Register and log in on FoSCoS with a username, password, and captcha.
- Step 3: Select "Apply for New License/Registration" from the dashboard.
- Step 4: Provide necessary details regarding premises and types of businesses (KoBs).
- Step 5: Complete Form B and attach required documents like Aadhaar Card, Form IX, and proof of premises.
- Step 6: Preview and submit the application, then proceed with payment.
- Step 7: Upon payment completion, receive a receipt with a 17-digit reference number for future use.
- Step 8: The Food Authority grants the license after scrutinizing the application and documents.
Form Names: Form I & II
FSSAI Approval Of Non-Specified Ingredients For Food Supplements
The food supplement industry is rapidly evolving with new innovations and ingredients. Global trade has made it easier to access novel products, but these may not be regulated by FSSAI. Therefore, it's crucial to register and obtain FSSAI approval for these products before manufacturing them in India for commercial use, under the category of Non-Specified Foods.
Regulatory Body Requirement
The Food Safety and Standards Authority of India (FSSAI) is the governing body responsible for approving all food products in India. This service aims to grant approvals for Non-Specified Ingredients/Products - for Food Supplements to manufacturers prior to product launch. The approval process typically spans from six to twelve months.
How to Apply
The applicant must follow these steps:
- Step 1: Prepare the dossier in the specified format, including necessary scientific information and study details.
- Step 2: Fill out Form-I, upload required documents, and pay the online fee of 50,000 rupees, GST inclusive.
- Step 3: The expert committee appointed by the Food Authority will conduct an initial review of the application and provided data. Any deficiencies will be notified to the applicant within 45 days of application receipt.
- Step 4: The FBO should address any required information within 30 days of receiving the notification.
- Step 5: After further evaluation and in accordance with FORM-II, the Food Authority may approve or reject the application.
- Step 6: Upon approval, the FBO must conduct and submit post-market surveillance data on relevant safety and efficacy parameters within one year of product placement.
- Step 7: If the application is rejected, the FBO may appeal to the CEO within thirty days of receiving the rejection notice.
- Step 8: In case of rejection by the appellate Authority, the FBO may file a review petition before the Chairperson of FSSAI within thirty days of the appellate order issuance.
- Step 9: The decision of the Chairperson shall be final.
Frequently Asked Questions
How are functional food supplements different from regular food supplements?
Are there any specific labeling requirements for functional food supplements?
Yes, functional food supplements must comply with labeling requirements set by regulatory authorities like the FSSAI.

How can manufacturers ensure the quality and safety of their functional food supplements?
Manufacturers should adhere to good manufacturing practices (GMP) and conduct rigorous testing for purity, potency, and safety.

What are the permissible health claims for functional foods?

What stability testing is required for functional food products?
Manufacturers should conduct shelf-life studies to determine the product’s stability over time. This involves assessing factors like temperature, humidity, and light exposure. The goal is to ensure that the product remains safe and effective throughout its intended shelf life.

Contact us
Please feel free to talk to us if you have any questions. We endeavour to answer within 24 hours.
