CDSCO
Regulatory Services for Importers
Seamless Entry, Regulatory Excellence: Your Partner in Navigating Medical Device Compliance for Import Success
Tap into India's booming medical device market! We navigate the revamped Indian regulations, empowering global companies to seamlessly penetrate this thriving landscape. Our expertise ensures compliance with updated laws, facilitating swift market entry. Join us to conquer opportunities in India's evolving healthcare arena effortlessly.
Regulatory framework explained
Overseas manufacturers wanting India entry in this business cannot apply directly to the Central Drugs Standard Control Organisation (CDSCO), the regulatory governor, for an import license. This has to be rooted through an authorized licensing agent, who in turn should possess a valid import license under Form 20B and 21B.
CliniExperts act as a medical device regulatory consultant and holds a valid import license (Form 20B and 21B) to assist foreign manufacturers to import their medical devices in India in less time and without any hassle. Hence speeding up their business ventures.
Devices classification:
- Class A: Low risk items such as absorbent cotton wool
- Class B: Low moderate risk items such as thermometers
- Class C: Moderate high risk items such as implants
- Class D: High risk items such as heart valves

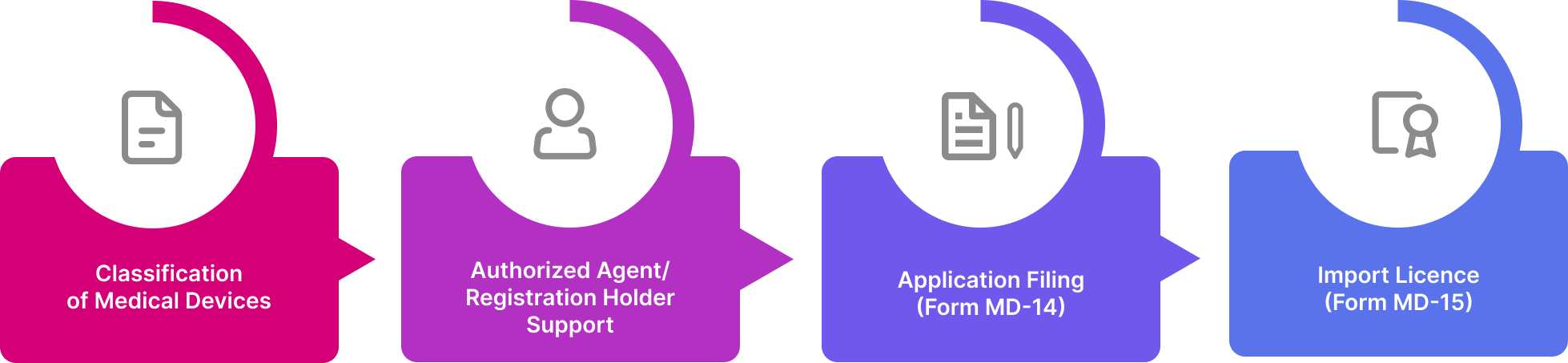
Import License Application Process
The process for granting licenses has been simplified and paced to allow quick launch of products in the market. The documentation and procedures have been aligned with global formats and conventions to unify and simplify procedures. Obtaining a medical device import license involves four key steps:



Regulatory Framework as per Classification of Products
Class A, B, C, D
Foreign manufacturers seeking to enter the Indian market in this industry cannot directly apply to the CDSCO, for an import license. Instead, they must go through an authorized licensing agent. The agent files application with CDSCO for import license under Form MD-14, and after due checks and verification, is granted permission under Form MD-15. This is applicable for all device classes.
New Medical Devices
For this category, a 3-layer permission process is applicable for all device classes:
- Clinical investigation permission, applied for under Form MD-22, and licensed under Form MD-23
- Import license applied for under Form MD-26, and granted under Form MD-27
- Test license applied for under Form MD-16, and granted under Form MD-17
Why Choose CliniExperts?
Invest in Trusted Excellence of CliniExperts to unleash the Potential of Indian Medical Device Manufacturing.
Contact our team to get the best support for regulatory services in India

Existing Devices
| Applicant | Risk/Class | Type of Licence | Forms |
| Importer | A,B,C,D | Importer Licence | Application: MD-14, Application: MD-15 |
New Devices
| Applicant | Risk/Class | Type of Licence | Forms |
| Importer | A,B,C&D | Clinical Investigation Permission | Application: MD-22, Application: MD-23 |
| A,B,C&D | Import Licence | Application: MD-26, Application: MD-27 | |
| A,B,C&D | Test Licence | Application: MD-16, Application: MD-17 |

CliniExperts – With You, All The Way
In 2018, around 159,98,7000 persons died due to poor quality of healthcare in India. These tragic numbers are expected to come down significantly with a reforms-based regulatory regime in place. And helping overseas companies sail smoothly through the regulatory process is CliniExperts, with its experienced and committed talent pool, latest know-how, and proven capabilities as a single-window regulatory service provider to medical devices importers.
Till date, we have fully serviced 206+ importers to their utmost satisfaction. Testimonies of just two of them will convince you to walk the talk with us.
Related Services
SUGAM REGISTRATION
Importer | Regulatory Body: CDSCO
An importer of Medical Devices who intends to get a registration/importing license can do the registration on the SUGAM portal.
The registration process for medical devices is primarily done on the SUGAM portal, a website where the applicants apply for approval of licenses, FSC and Registration numbers.
AUTHORIZED AGENT
Importer | Regulatory Body: CDSCO
A foreign company who doesn’t have its establishment in India, wants to enter India market, then they can appoint an authorized agent to register and market their products in India. An “Authorized Agent” means a person or entity in India authorized by the foreign company.
The authorized agent will be responsible for the import and business activities of the foreign company in India including compliance to the provisions of the Drugs and Cosmetics Acts in all respects.
IMPORT LICENSE APPLICATION AND APPROVAL
Importer | Regulatory Body: CDSCO
India has emerged as the world’s top manufacturer of medical devices over the past two decades. The Central Drugs Standards Control Organization (CDSCO) is authorized regulatory to approve applications to grant the permission to import medical devices in India. The Medical Device import license can be obtained by filling Form MD-14 and submitting to the CDSCO.
IMPORT LICENSE FOR NEW MEDICAL DEVICES
Importer | Regulatory Body: CDSCO
Central Drugs Standard Control Organization (CDSCO) is the regulatory authority of India that grants the permission to import new medical device in India- Form MD 26 & 27. To get an import/manufacturing license for the sale/distribution of a medical device that does not have a predicate medical device, the manufacturer/importer must make an application to the Central Licensing Authority. The import license applicant must do this via an authorized agent having a manufacturing or wholesale license for the sale or distribution of medical devices.
Frequently Asked Questions
Is it possible for multiple importers to handle the same product?
Yes, there can indeed be multiple importers for the same product, provided that all applicants meet the criteria outlined for submitting their applications.

What are the fees for a manufacturing site if importers wish to register devices belonging to multiple classes (A, B, C, or D)?
According to the Second Schedule, manufacturers are required to submit specific fees for different classes of medical devices. If an organization manufactures products across multiple classes, the fees associated with the highest class among them need to be paid.

If the first importer has obtained approval for a new investigational medical device, do subsequent applicants also need approval, or can they simply apply for an import license?
If the initial importer has already obtained approval for a new investigational medical device, subsequent applicants can proceed by solely applying for the import license for the same medical device without the need for additional approval.

Why would I require authorized agent support?
For a foreign company aiming to market its product in India, the primary requirement is the registration of products through an Indian establishment. Foreign companies lacking an Indian establishment or local partners must engage an authorized agent to manage the import of products into India on their behalf.

How will I benefit from appointing you as my authorized agent instead of my existing distributor in India?
To kickstart operations in India, only a single Indian establishment is required. If you opt to change your distributor after initial registration, the registration process would need to be repeated. However, by partnering with CliniExperts, registration is a one-time process. This allows flexibility to add or switch distributors as necessary without going through the registration procedure repeatedly.

Contact us
Please feel free to talk to us if you have any questions. We endeavour to answer within 24 hours.
