NOC for Form 29 in India - CT-10 & CT-11

Do you wish to quickly get a NOC for Form 29 In India to manufacture test batches for test and analysis? Your one-stop shop for successfully handling the crucial NOC under Form 29 licensing process is CliniExperts. Choose us as your partner to ensure a smooth transition to commercial success. Contact us to learn how we can help you earn your license.
NOC for Form 29 – Overview

Who Can Apply?
Any person who intends to manufacture a new drug or an investigational new drug in order to conduct a clinical trial, a bioavailability and bioequivalence study, or an examination, test, and analysis must submit an application in Form CT-10 to the Central Licensing Authority for the permission.
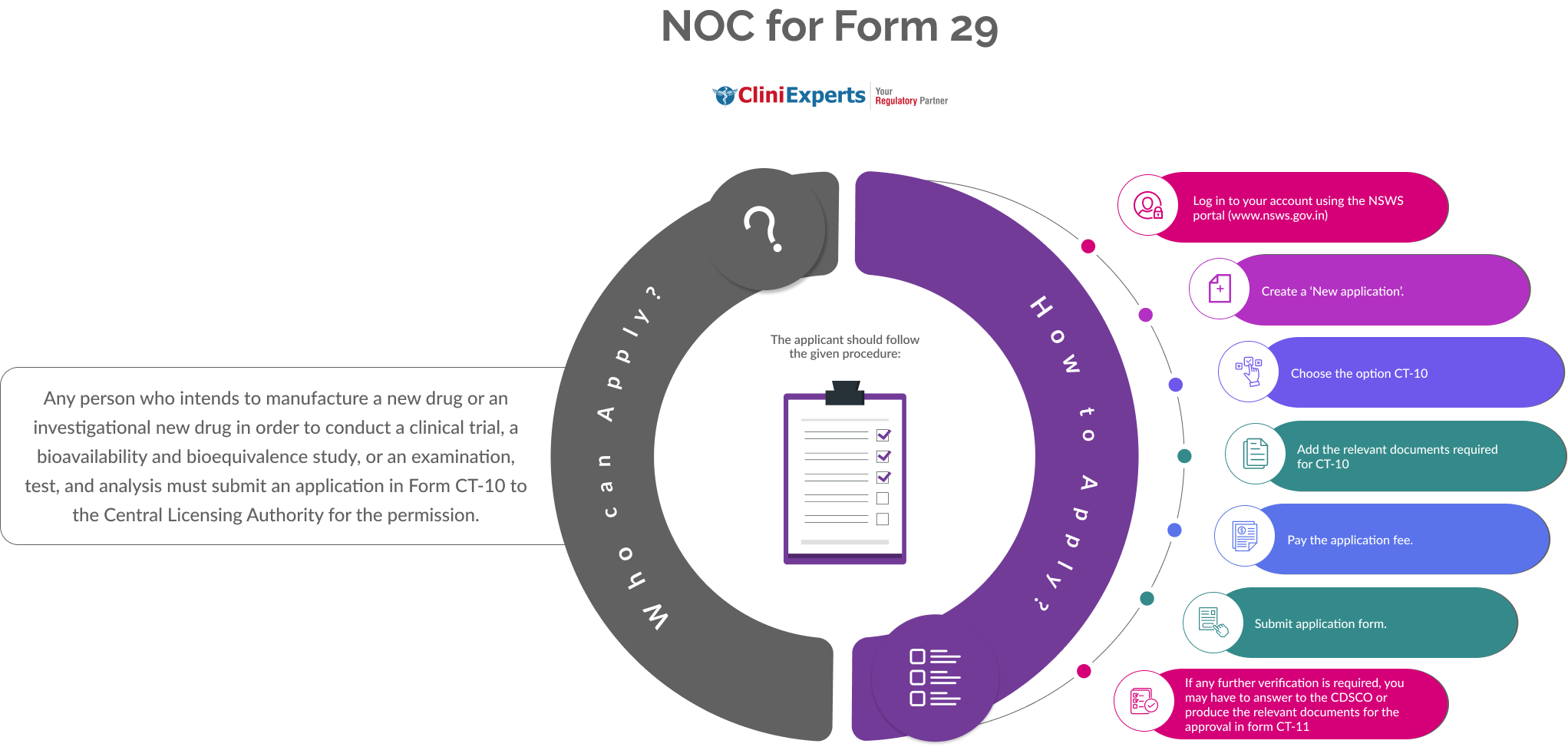
How To Apply?
The Applicant must follow the following process:
-

Log in to your account using the NSWS portal (www.nsws.gov.in).
-

Create a ‘New application’.
-

Choose the option CT-10.
-

Add the relevant documents required for CT-10.
-

Pay the application fee.
-

Submit application form.

Validity
The NOC for Form 29 issued under CT-11 shall remain valid for a period of 3 years from the date of issue, unless suspended or canceled by the Central Licensing Authority.
Fee Involved
The fee of licenses for test and analysis of a drug has been kept 5000 per drugImportant Documents

During the documentation process, the following documents need to be submitted:
- Manufacturer details
- Detailed utilization break-up for each drug indicating the nature of tests and quantity required for each test
- Copy of valid manufacturing license
- Proposed SOP for manufacturing
- Proposed SOP for testing/Analysis
- List of equipments and testing facility
- Proposed specification and STP
- Source and specification of active raw material for formulation
Timeline to get
CT-11
from Central Drugs Standard Control Organisation
7
Working DaysEssential Tips
Manufacturers need to follow all CDSCO criteria for biological product registration, to successfully get a registration certificate for their testing, analysis and examination facilities. Key factors to keep in mind while making this application include:
- The applicant should use the biological manufactured under the license only at the place mentioned in the license. The license should exclusively be used for the purpose of examination, analysis or test.
- The applicant should allow any appointed 4 Inspectors to enter the premises where the drugs are manufactured to ensure only examination, analysis or test work is being conducted.
- The applicant should keep a record of the quantity of biologicals manufactured for examination, analysis or testing. The applicant should also provide any names of persons to whom the drugs have been supplied.
Expert Advise
According to our experts, ensure the following to avoid any delays or cancellation of your license:
- Strict adherence to the CDSCO guidelines.
- Use the biological manufactured only at the specific place mentioned in the license.
- Allow any 4 inspectors to enter the premises anytime and monitor that the investigational new drug is only used for examination, analysis and test work. The applicant should also provide names of people to whom the drugs are supplied.
Frequently Asked Questions
Can the form 11 license be renewed?
No. There is no provision for the renewal of Form 11 license once it is expired. The applicant has to apply for a fresh form11 license with challan and other essential documents.

Can the drugs imported under Form 11 be used for animal studies?

How many drugs can be imported under a single Form 11 license?
A maximum of 10 drugs can be imported under a single Form 11 license.

