PHARMACEUTICAL QUALITY AND COMPLIANCE SERVICES
Quality GxP services for pharmaceutical manufacturers for the Indian and global markets.
TO LEARN MORE, CONNECT WITH US
What Are GxP Services?
GxP is a set of quality and regulatory guidelines that work towards ensuring the safety of pharmaceutical products in a systematic manner while maintaining the processes' quality throughout manufacturing, control, storage, and distribution.
The variable “x” in GxP stands for varied fields according to the application of standards - Good Manufacturing Practice (GMP), Good Clinical Practice (GCP), Good Laboratory Practice (GLP), and more.
GxP regulations and guidelines are global for regulated industries. Thus, every regulated industry, be it food, pharma, or medical devices, must meet regulatory compliance with bodies, such as the Food and Drugs Administration (FDA) for the United States, the Medicines and Healthcare Regulatory Agency (MHRA) for the United Kingdom, the European Medicines Agency (EMA) for the European Union, and the Central Drugs Standard Control Organization (CDSCO) for India.
Why Manufacturers Need GxP Services
GxP (Good Practice) services are critical for manufacturers worldwide as they help ensure the products manufacturers deliver are safe, effective, and of high quality. Compliance with GxP regulations is a shared responsibility across all organisational levels, from manufacturing to distribution. Implementing a strong pharmaceutical quality system that incorporates GxP guidelines helps prevent issues, such as drug shortages, counterfeit products, and regulatory non-conformities, and further builds public trust.
A GMP Design Review guarantees that your project aligns with quality standards, minimizes risks, and can be executed, monitored, and controlled efficiently. This process represents a cost-effective and high-value investment to ensure the excellence of your new facility or upgrade.
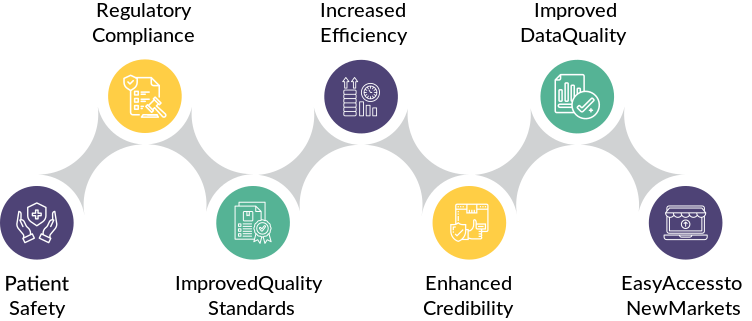
Benefits of GxP Compliance for Manufacturers
GxP Services for Manufacturers Supplying Products to the Indian and Global Market
Are you a pharmaceutical manufacturer struggling with the evolving regulatory landscape in India, EU, UK and USA?
CliniExperts GxP Services can help you navigate these challenges with ease.
GxP Services Benefits for Manufacturers
GxP Services for Manufacturers Supplying Products to the Indian Market
Schedule M guidelines for Good Manufacturing Practices (GMP), devised by the Ministry of Health and Family Welfare (MoHFW), in India are part of the Drugs and Cosmetics Rules, 1945. They define the GMP requirements for pharmaceutical manufacturing and are periodically updated to align with international standards and address the evolving needs of the industry.
CliniExperts Quality and Compliance Services helps identify gaps and develops CAPA plans to ensure compliance with the revised Schedule M requirements.
Some recent developements in Schedule M are:
- Harmonization with international standards
- Emphasis on Quality Management Systems
- Stricter requirements for facilities and equipment
- Documented validation of manufacturing processes and protocols
- Digital record keeping and data management
GxP Services Offered by CliniExperts
 |
Development and Support for Quality Systems in Pharmaceuticals |
Our consultants help you build compliant quality systems to drive product quality and operational efficiency with:
- Quality Management Systems, SOPs, and quality manuals
- Staff training and audit preparation
- Performance metrics, CAPAs (Corrective and Preventive Actions)
- Risk management
- Quality Management Software implementation
 |
Creation of GMP Strategies |
Our expert guidance helps pharmaceutical companies develop effective GMP strategies, ensuring product quality and regulatory compliance. We do:
- Design Quality Management System (QMS)
- Consulation on facility layouts and process validation
- Conduct risk assessments, develop mitigation strategies
- Design training programs for staff on GMP practices
- Prepare for internal and external audit
 |
Audits and Quality Assurance Service |
Our consultants help with internal and external audits and quality assurance in the pharmaceutical industry. We do:
- Conduct gap analyses and review documentation
- Plan and perform internal audits, identify issues, and provide improvement recommendation
- Develop corrective and preventive action plans (CAPAs)
- Enhance quality assurance systems
- Conduct mock inspections
 |
Mock Inspections, GAP Analysis, and Inspection Readiness Assessments |
Our consultants help you identify and rectify issues before official inspections, reducing the risk of regulatory non-compliance through:
- Conduct mock inspections that mimic regulatory audits
- Identify compliance issues and non-conformities and provide feedback
- Identify discrepancies in processes and existing quality systems
- Inspection readiness assesments by reviewing systems and documentation
- Train staff so they can respond to auditor inquiries
 |
Remediation Programs |
Our consultants help pharmaceutical companies by identifying compliance issues and developing remediation plans by:
- Investigate root cause of compliance issues
- Develop remediation action plans to address identified issues
- Train staff on new procedures and compliance requirements
- Track progress and verify the effectiveness of remediation efforts
- Provide feedback to strengthen GMP practices
 |
Compliance for the Early stages of Drug Development |
Our Consultants extend critical support in assessing the efficacy of the intended Drugs under development to ensure compliance and preparedness. We assess:
- The R&D Pilot Laboratory scale for NCE
- Compounding dispensary for the IND and NDA
- Pre-clinical laboratory and animal houses, including the BE and BA research organizations
- Human safety of the IMD and NDA at clinical research organizations
- Offer recommendations to bridge gaps and align practices with GCP requirements and industry best practices
 |
Quality 6-system Process Optimization and Throughput Enhancement |
Our consultants enhance the QMS with an optimal approach and automation to achieve operational excellence and resolve human barriers and lacuna. We do:
- Operational due diligence assessment of the business model and operation
- Map the 6-system process to identify the biases in the quality systems
- Identify discrepancies in company strategy for QbD, QRM, contamination control, documentation, and compliance practices.
- Track progress and verify the effectiveness of remediation efforts
- Bridge gaps and align practices by churning KPI and industry best practices
Looking For A Complete Solution?
At CliniExperts, our certified and pharmaceutically trained professionals are at the forefront of GxP standards. Leveraging our extensive expertise and international experience, we effectively address complex quality requirements through GMP-compliant design, tailored solutions, comprehensive quality documentation, project validation, and GxP compliance with regulatory bodies, including CDSCO, EU, MHRA, US FDA, PICS, and GMP.
Whether it is Good Manufacturing Practice (GMP), Good Clinical Practice (GCP), Good Laboratory Practice (GLP), or other GxP standards, we will connect you with industry experts proficient enough to guarantee seamless GMP compliance and other particular needs.
Our consultants assist pharmaceutical clients in achieving and maintaining GMP compliance throughout a product's lifecycle by providing tailored compliance services, including identifying compliance issues, developing and implementing remediation plans, providing training, and ensuring effective resolution and continuous improvement.

Certifications and Accreditations
With these certifications, we demonstrate our dedication to quality, safety, and customer satisfaction.

ISO 9001

ISO 27001

ISO 13485

Wholesale License
Related Service
COSMETIC LABEL COMPLIANCE
Importer | Regulatory Body: CDSCO
With a boom in the cosmetic market, it is essential to make your product such that it stands out from other competitors. A cosmetic product is also assessed by its consumers based on its label. Hence, a label should be appealing in a manner as well as per the regulations prescribed by the New Cosmetics Rules 2020 for quick approval from authorities. We at CliniExperts, handle your product label with the utmost precision. From assessing the label according to the standards and assisting you with the application process, our team of CliniExperts will ensure that your label artworks meets all laid out regulations and help with a speedy and hassle-free regulatory approval for a quick product launch.
LEGAL METROLOGY LABEL COMPLIANCE
Importer | Regulatory Body: CDSCO
With a boom in the cosmetic market, it is essential to make your product such that it stands out from other competitors. A cosmetic product is also assessed by its consumers based on its label. Hence, a label should be appealing in a manner as well as per the regulations prescribed by the New Cosmetics Rules 2020 for quick approval from authorities. We at CliniExperts, handle your product label with the utmost precision. From assessing the label according to the standards and assisting you with the application process, our team of CliniExperts will ensure that your label artworks meets all laid out regulations and help with a speedy and hassle-free regulatory approval for a quick product launch.
