Table of Contents
Cosmetic Label Compliance India : A Guide to Compliance

Introduction
Looking for Cosmetic Label Compliance India? Are you a cosmetic manufacturer or importer navigating the complex world of Indian regulations? Ensuring your product labels comply with the latest cosmetic safety and labelling guidelines is crucial for market access and consumer trust. In today’s competitive market, packaging and labelling serve not only as an information source for consumers but also as a marketing tool. However, ensuring your product labels comply with the latest standards is crucial for market access and consumer trust. This comprehensive guide breaks down the key cosmetic labelling requirements in India, providing you with the knowledge you need to stay compliant and avoid costly penalties. We’ll cover everything from mandatory declarations to specific rules for imported cosmetics, drawing from the Cosmetics Rules 2020, the Drugs and Cosmetics Act, 1940 and other relevant guidelines. It is important to keep in mind the Consumer Protection Act, particularly the consumer’s right to information and consumer education.
The Importance of Compliant Cosmetic Labels in India
Compliant cosmetic labels in India are crucial for ensuring consumer safety, regulatory adherence, and transparency in product claims. They play a vital role in the following aspects:
- Consumer Safety: Accurate labelling informs consumers about ingredients, potential allergens, and proper usage, reducing risks of adverse reactions. Misleading or incomplete labelling can result in health hazards, making compliance essential.
- Regulatory Compliance: Cosmetics must meet the labelling standards set by the CDSCO (Central Drugs Standard Control Organization), similar to how the FD&C Act in the U.S. prohibits misbranded or adulterated products. Failure to comply may lead to product recalls, fines, or even market bans.
- Market Access: Transparent and compliant labelling enhances credibility, helping brands gain consumer trust and maintain a competitive edge in India’s growing cosmetics industry. Proper labelling ensures that products are not deemed misleading under legal scrutiny.
- Legal Requirements: The Drugs and Cosmetics Act, 1940 mandates specific declarations such as manufacturer details, net quantity, and product claims. Non-compliance can result in legal consequences, including fines, license suspension, or criminal prosecution. Misleading claims or failure to disclose material facts can also classify a product as “misbranded.”
- Prevent Consumer Exploitation: Labels must not be deceptive or omit critical information. Misleading packaging such as incorrect claims about product benefits or unclear quantity declarations could exploit consumers. Similar to the U.S. Fair Packaging and Labelling Act, accurate labelling ensures fair value comparisons and informed purchasing decisions.
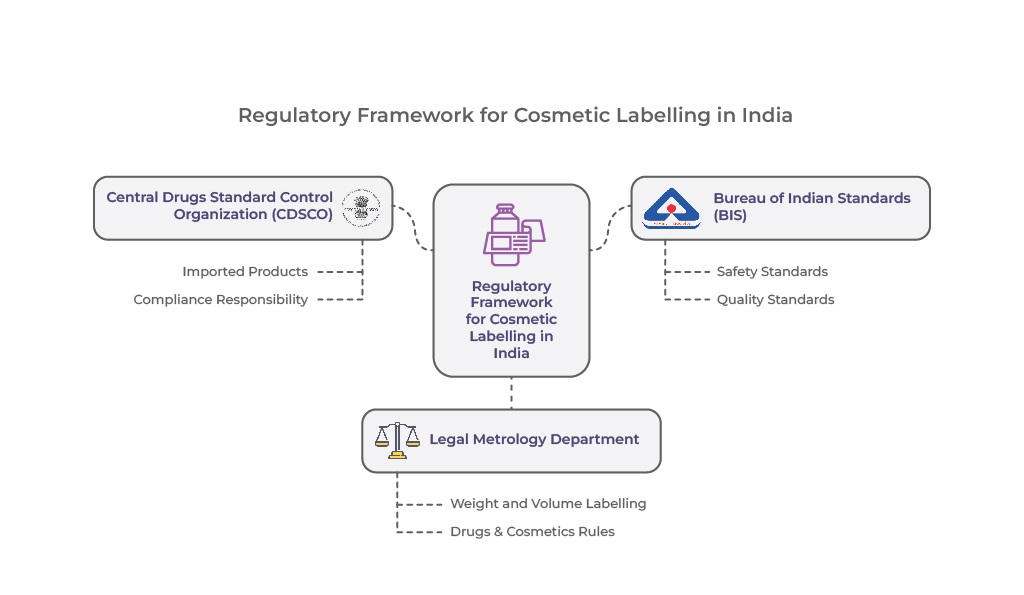
Key Regulatory Bodies Governing Cosmetic Labelling
The regulatory bodies that oversee cosmetic labelling in India to ensure compliance with safety, quality, and transparency standards are:
- Central Drugs Standard Control Organization (CDSCO): The CDSCO plays a key role in regulating cosmetics in India, particularly overseeing imported products, while State Licensing Authorities (SLA) manage domestically manufactured cosmetics. Unlike pharmaceuticals, CDSCO does not pre-approve cosmetic labels during marketing authorization, making manufacturers and importers responsible for ensuring compliance.
- Bureau of Indian Standards (BIS): BIS sets safety and quality standards for cosmetics in India, covering both locally produced and imported products. Adherence to BIS standards for cosmetics in India ensures product reliability and consumer safety.
- Legal Metrology Department: Cosmetics in India must comply with the Legal Metrology (Packaged Commodities) Rules, 2011, which mandate accurate weight and volume labelling requirements for cosmetics in India. Additionally, labelling must align with the Drugs & Cosmetics Rules under the Drugs and Cosmetics Act, supplemented by relevant BIS guidelines.
Indian Regulatory Requirements for Cosmetic Products
The regulatory landscape for cosmetic products in India is governed by stringent guidelines mentioned below to ensure safety, quality, and efficacy:
- Location and Environment: The manufacturing premises should be situated in a clean and hygienic environment. Proper sanitation must be maintained throughout the premises, and the manufacturing area must be well-ventilated and kept clean.
- Building Specifications: The walls of the manufacturing area must extend at least 6 feet from the floor, and the surfaces should be smooth, waterproof, and easy to clean to facilitate safe and efficient production activities.
- Water Supply and Wastewater Management: Only potable water should be used in the production process. Additionally, an effective system for the disposal of wastewater must be in place.
- Staff Health and Sanitary Standards: All employees must be free from infectious or communicable diseases. They should be provided with appropriate uniforms, gloves, and masks when necessary to maintain hygiene standards.
- Cosmetic Manufacturing License: A regulatory review of the product’s details, including its quality, safety, and compliance with BIS standards, is required for the manufacturer to obtain a cosmetic license.
Mandatory Declarations on Cosmetic Labels: What You Need to Know
The Cosmetics Rules, 2020 mandate specific mandatory declarations for cosmetics for both domestically manufactured and imported cosmetics, making it the responsibility of manufacturers and importers to comply. Unlike some other markets, India’s regulatory bodies like the State Licensing Authority (SLA) for domestic products and the Central Drugs Standards Control Organization (CDSCO) for imports do not pre-approve product labels during the licensing process. Instead, compliance is verified post-market, making it essential to get labelling right before a product reaches consumers.
The following mandatory cosmetic product labelling requirements must be displayed on all cosmetic labels:
- Product Name and Description: Clearly state the product’s identity and intended use.
- Name & address of Manufacturer: Indicate “Manufactured by….” Or “Packed by…”, complete address of the premises where the product was manufactured, with name. Additionally, include the packer/importer information (if applicable).
- Ingredient List: List all ingredients, along with their respective percentages, in descending order of weight or volume.
- Retail Sale Price: Clearly mention the MRP of the product. For reducing the MRP, a sticker with revised MRP may be affixed inclusive of all taxes. Such a sticker should not cover the MRP declaration by the manufacturer or packer on the package.
- Net Quantity: Declare the net weight or volume in metric units, following weight and volume labelling requirements for cosmetics in India.
- Batch Number and Manufacturing Date: Essential for tracking and quality control. You may also need a code number on cosmetic labels for traceability.
- Expiry Date: Clearly indicate the product’s shelf life using “Use before … (Month and year)” or provide the Expiry date.
- Usage Directions: Include instructions for safe use and cautionary warnings.
- Import Details (for Imported Cosmetics): Include the name and address of the importer.
- Consumer Care Details: Provide the manufacturer name and address on cosmetic labels in India. Additionally, provide the telephone number and email address of someone who can be contacted for consumer complaints.
A cosmetic product typically has a label on the container (inner label), an outer wrapper or box (outer label), and sometimes a leaflet. The Cosmetics Rules 2020 specify where certain declarations must appear.
- Information Required on BOTH Inner and Outer Labels:
- Name of the cosmetic
- Name of the legal manufacturer
- Complete address of the manufacturing premises
- “Use before” including Month and year or the Expiry date
- List of ingredients (over 1% concentration in descending order, followed by those <=1% in any order, preceded by “INGREDIENTS”)
- Information Required on EITHER Inner OR Outer Label:
- Distinctive batch/lot code (preceded by “Batch No.,” “B. No,” etc.)
- Manufacturing License Number (preceded by “M,” “M.L. No.,” or “Mfg. Lic. No.”). It’s advisable to include these on both labels as consumers often throw away the outer packaging.
- Information Required ONLY on the Outer Label:
- Net contents (weight for solids, fluid measure for liquids, or either for semi-solids).
- Number of items if more than one.
- Information Required ONLY on the Inner Label:
- Adequate directions for use (if hazards exist).
- Any warnings, cautions, or special directions.
- Names and quantities of hazardous or poisonous ingredients (if applicable).
If there is only one label, it must contain all the required information.
Note:
- Declarations are to be either in Hindi or English (in addition to this, other local languages are also allowed).
- If declarations are in the form of handwriting or hand-script, such declarations should be clear, unambiguous, and legible.
- A label for making the declarations can be affixed to imported packages.
- Retail sale price and net quantity should be painted, printed, or inscribed in contrasting colors.
Specific Labelling Requirements for Cosmetics
Specific labelling requirements for cosmetics in India are outlined to ensure consumer safety, product transparency, and regulatory compliance, with clear guidelines on the information that must appear on specific product categories.
1. Labelling Requirements for Imported Cosmetics in India
Imported cosmetics must adhere to all requirements for domestically produced cosmetics, except that if the country of origin does not require the manufacturing license number to be mentioned, it is not required in India either. Modifications to labelling can occur at a customs-bonded warehouse before clearing Indian customs. The following CDSCO guidelines for imported cosmetic labelling ensure compliance:
- Labels must be in English or Hindi.
- Clearly state the country where the product was manufactured.
- Provide the name and address of the importer in India.
- Include the Import Registration Certificate number (preceded by “RC,” “RC No.,” etc.). If the manufacturer’s site is not declared, the country of manufacture must be stated (“Made in (Country)”).
2. Exemptions for Small Cosmetic Packages
For compact packaging, some labelling relaxations apply:
- If the container holds ≤ 60 ml liquid or ≤ 30 g solid/semi-solid, the manufacturer’s address can be abbreviated to the principal location and pin code.
- Batch codes are not required for products ≤ 10 g (solids/semi-solids) or ≤ 25 ml (liquids). Instead of a batch number for soaps, the month and year of manufacture may be given.
- A net content declaration is not needed for perfumes, toilet waters, or similar products ≤ 60 ml, or solid/semi-solid products ≤ 30 g.
- Ingredient lists can be omitted for products ≤ 60 ml liquid or ≤ 30 g solid/semi-solid.
3. Special Labelling for Hair Dyes and Toothpastes
Due to their chemical composition, hair dyes and toothpastes require additional cautionary statements in both English and the local language on inner and outer labels:
For hair dyes:
- Warning: This product may cause skin irritation. Conduct a patch test before use. Do not use it for dyeing eyebrows or eyelashes, as this may cause blindness.
- Allergy Test Instructions: Apply a small amount behind the ear or on the forearm. Wait 24 hours and check for irritation before full application.
For toothpastes:
- The fluoride content in toothpaste must not exceed 1000 ppm. Additionally, the fluoride concentration, measured in ppm, and the date of expiry should be clearly indicated on both the tube and the carton.
4. Heavy Metal and Hexachlorophene Declaration
Cosmetics should include a declaration on their labels that specifies that their product contains heavy metals within the allowable limits and no hexachlorophene. The Drugs and Cosmetics Act prohibits the manufacturing and import of cosmetic products that exceed the prescribed limits of these substances.
- Provision for soaps:
- In the case of soaps, hexachlorophene may be used in concentrations not exceeding 1% by weight. A cautionary note must be printed on the packaging, clearly stating: “Contains hexachlorophene – not to be used on babies.” This declaration should be prominently displayed on the wrapper of each soap product.
5. Additional Labelling Requirements
- Vegetarian/Non-Vegetarian Dot: For soaps, shampoos, toothpastes, and other cosmetics and toiletries, include a brown dot for non-vegetarian origin and a green dot for vegetarian origin.
- Non-Standard Pack Size Declaration: If a cosmetic is packed in a size other than those specified in the Second Schedule of the Legal Metrology (Packaged Commodities) Rules, 2011, it should be prominently labelled as “Not a standard pack size under Legal Metrology (Packaged Commodities) Rules, 2011”.
- Animal Testing Declaration: While animal testing is banned in India, brands may voluntarily include a cruelty-free statement. However, certification logos (e.g., PETA’s ‘Beauty without Bunnies’) should only be used if officially obtained.
6. Label Modifications Post-Manufacture
Once a cosmetic product leaves the factory (domestic) or clears customs (imported), any changes to mandatory declarations require prior approval from the Drugs Controller General of India (DCGI) and relevant regulatory authorities.
7. Restrictions and Prohibitions
CDSCO guidelines for cosmetic label claims help ensure accuracy and prevent misleading information:
- Avoid making false or exaggerated claims about your product’s efficacy.
- Understand the prohibition against false or misleading claims on cosmetic labels in India.
- Stick to approved claims for cosmetic labels in India and avoid making unsubstantiated assertions.
- Be aware of restricted words for cosmetic label claims in India to prevent regulatory issues.
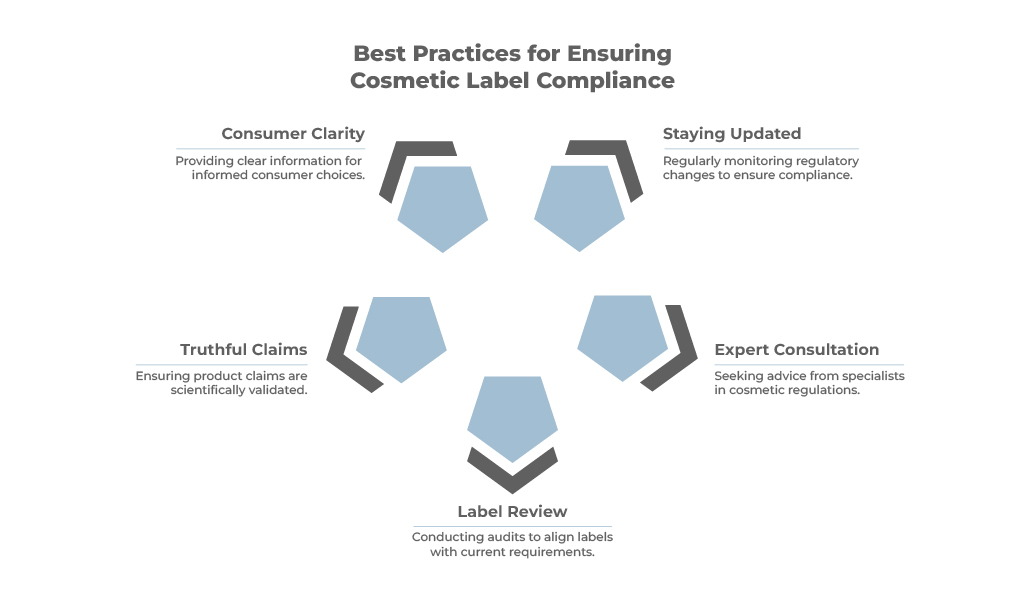
Best Practices for Ensuring Cosmetic Label Compliance
Ensuring that Indian cosmetics labelling guidelines are duly fulfilled is crucial for meeting regulatory standards, protecting consumer safety, and maintaining brand integrity. Adopting these best practices can help manufacturers through complex regulations and avoid potential legal and market risks.1,9
- Stay Updated: Cosmetic regulations are subject to change. Regularly consult the CDSCO website and industry publications.
- Consult with Experts: Seek guidance from regulatory consultants specializing in Indian cosmetic regulations.
- Review and Audit: Regularly review your labels to ensure they meet the latest requirements.
- Truthful & Transparent Claims: Ingredient benefits should not be falsely transferred to the final product unless scientifically validated.
- Evidential Support: All claims must be backed by reliable, reproducible testing methods to ensure accuracy and compliance.
- Honesty in Advertising: Avoid overstating product benefits, claims must reflect tested and proven properties.
- Fair & Accurate Representation: Do not denigrate competitors or legally approved ingredients; maintain objective, verifiable messaging.
- Consumer Clarity: Labels should provide clear, understandable information, enabling consumers to make informed choices without confusion or misinterpretation.
Expert Advice on Cosmetic Labelling Compliance: Key Dos and Don’ts
Here’s our expert advice to ensure cosmetic label compliance in India with labelling regulations:
- Ensure all declarations are clearly displayed on the Principal Display Panel (PDP) for visibility and compliance.
- The text on labels should be legible, prominent, and easy to read to avoid confusion and ensure consumer safety.
- Follow the prohibition of misleading claims on cosmetics for transparency.
- Manufacturers or individuals are prohibited from altering, obliterating, or defacing any inscriptions or marks on the container, label, or wrap.
- Stickers should not be used to alter or make declarations on labels, except when reducing the Maximum Retail Price (MRP).
- If using stickers to reduce MRP, ensure that the MRP declaration made by the manufacturer/packer remains visible and is not covered.
Conclusion
Navigating Indian cosmetics labelling guidelines may seem challenging, but with a clear understanding of the regulations and a commitment to compliance, you can confidently bring your products to the Indian market. By prioritising accurate and transparent labelling, you not only meet legal requirements but also build trust with your consumers, ensuring long-term success. Staying informed about compliance with Indian cosmetic regulations, including the Cosmetics Rules 2020, is essential for avoiding penalties and fostering consumer confidence. Remember, the consumer’s right to information should be at the forefront of your packaging and labelling efforts. With dedication to these standards, you ensure that your brand remains reputable, and your products are safe, reliable, and well-received in the marketplace.
Call to Action
Need help ensuring your cosmetic labels comply with Indian regulations? Contact CliniExperts for a free consultation today! We provide tailored guidance on all aspects of cosmetic labelling, from ingredient listings to packaging details. Our expert team will help you with the complex regulatory landscape, ensuring your labels meet all required standards.
References
- Provided reference document.
- U.S. Food and Drug Administration. Cosmetics Labelling Regulations: Cosmetics Labelling Guide. Available from: https://www.fda.gov/cosmetics/cosmetics-labelling-regulations/cosmetics-labelling-guide
- Kavya, Himmat Singh Chawra. Regulatory Provisions for Cosmetics in India. Research Journal of Topical and Cosmetic Sciences. 2022; 13(1):14-0. doi: 10.52711/2321-5844.2022.00003
- Central Drugs Standard Control Organization. The Cosmetics Rules, 2020. Available from: https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=OTIyNg==
- Central Drugs Standard Control Organization. Guidance document for cosmetics. Available from: https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf-documents/cosmetics/Guidancedoccos.pdf
- Legal Metrology (Packaged Commodities) Rules, 2011. Available from: https://megweights.gov.in/acts/Legal-Metrology-Packaged-Commodities-Rules-2011.pdf
- Fischer J. Green and/or brown: Governing food production in India. Res Globalization. 2020;2:100017. doi: 10.1016/j.resglo.2020.100017. Available from: https://www.sciencedirect.com/science/article/pii/S2590051X2030006X
- Central Drugs Standard Control Organization. Frequently asked questions on cosmetics [Internet]. New Delhi: Central Drugs Standard Control Organization. [cited 2025 Feb 17]. Available from: https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf-documents/cosmetics/FAQs-Cosmetics_New.pdf
- Lionetti, Nicola & Rigano, Luigi. (2018). Labelling of Cosmetic Products. Cosmetics. 5. 10.3390/cosmetics5010022.
Recent Posts
Organic Food Labelling In India| Certification, and Import of Organic Food in India

This Article is All About Organic Food Labelling In India and Certification, and Import of Organic Food in India. Explained in Detail About What is Organic Food labelling? Summary Short Description Wi..
Clinical Investigation Approvals: An Overview of Forms MD-22 and MD-23

Summary Short Description Strict regulatory protocols govern clinical investigations for medical devices. Central to this process are forms MD-22 and MD-23. Form MD-22 is an application to Central Lic..
HAVE A QUERY?
REACH US!Office
New Delhi
Unit No. 324 & 325, City Centre Mall, Plot No. 5, Sector 12, Dwarka, India - 110075
+917672005050
Bengaluru
RMZ Galleria, 1st floor, Ambedkar Colony, Yelahanka, Bengaluru, Karnataka, India – 560064
Call us on
Sales: +91 7672005050
Reception: +91-11-45214546
Timings
9 am to 6 pm (Monday to Friday)


