Cosmetic Manufacturing License in India - Cos-5, Cos-6 & COS-8, Cos-9

CliniExperts has been proved to be the best regulatory consultant for providing the Cosmetic Manufacturing Licenses in India. Our professional assistance ensures a smooth and speedy process. From documentation to regulatory steps, we're here to simplify the process and help you navigate every stage with ease.
Cosmetic Manufacturing License (Form COS-8) – Overview
The regulatory body governing manufacturing license for cosmetics is State Licensing Authority (SLA). The SLA ensures that cosmetic manufacturers meet all the requirements for their products.
To manufacture cosmetics in India, it's must for any company to possess a valid Cosmetic Manufacturing License. So, they should make an application in Form COS 5 for grant of License and COS 6 for a loan license for the sale and distribution of cosmetic products. The permission is obtained in COS 8 and COS 9.
The application process involved in this is as follows:- The applicant must submit the application (COS 5 or 6) through the SLA Online portal.
- The applicant should check that all the submitted documents align with the checklist.
- Upon completing the submission of all documents, the SLA scrutinizes the application. It then approves the grant or loan license in Form COS 8 or 9 for manufacturing cosmetics in India or it may reject it.
- The timeline to obtain the grant of License could take forty-five days from the date of application.
Who Can Apply?
An applicant must possess the following criteria for applying for Cosmetic Manufacturing License:
- The applicant must hold the ownership or rental or other basic documents.
- The factory premise should comply with all the requirements specified in the Seventh schedule.
- The factory should be positioned in a sanitary place and not be connected to residential areas.
- The cosmetic manufacturer should conduct under the directions of competent technical staff.
- There should be at least one full-time employee and essentially have a Diploma in pharmacy or registered under the Pharmacy act, 1948 etc.
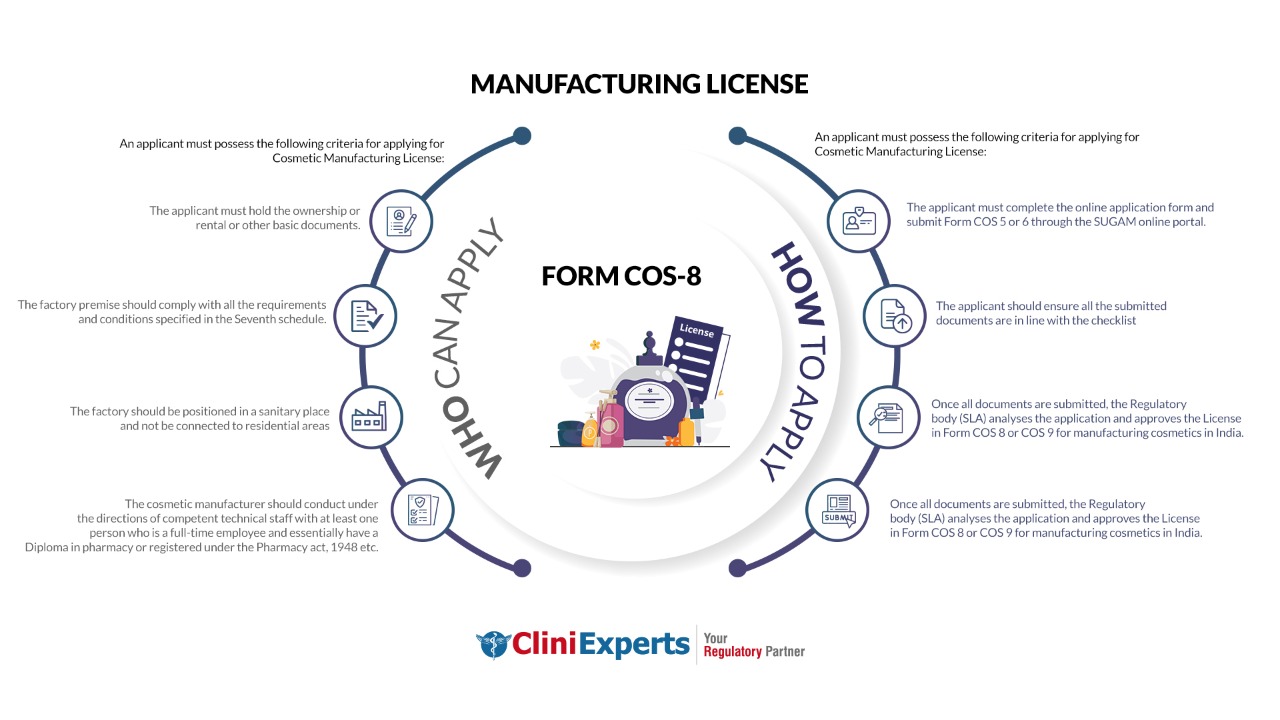
How To Apply?
The Applicant must follow the following process:
-

Applicant must register him/herself in Sugam portal to create an ID.
-

The applicant must complete the online application form and submit Form COS 5 or 6 through the SLA online portal or at SLA office (for offline mode).
-

The applicant should ensure all the submitted documents are in line with the checklist.
-

The Regulatory body (SLA) inspects the premises, analyses the application and approves the License in Form COS 8 or COS 9 for manufacturing cosmetics in India.
-

The timeline to obtain the grant of License could take forty-five days from the date of application.

Validity
The validity of the Cosmetic Manufacturing License (COS 8/COS 9) is 5 years.

Fee Involved
The Government charges a fee of Rs. 10000 for the grant of License in Form COS-8 for manufacturing cosmetics for sale and distribution of ten items of each category of cosmetic. A fee of RS. 500 is also charged for each additional item in the cosmetic category.Important Documents

The following are a few major documents needed for the application process:
- Ownership document
- A copy of the approved layout plan
- Rent agreement and purchase document
- Self-certificate of compliance with the Good Manufacturing Practice
- List of machinery and equipment
- List of Cosmetics with Composition formula and labeling
- List of Directors
- Applicant and technical person’s ID proofs
- COA, MOA, AOA, COI
- Technical person documents
- Receipt of fee
Timeline to get
COS-8, Cos-9
from State Licensing Authority
45
DAYSEssential Tips
The following main points should be considered during the application process. The applicant must have
- Complete labelling according to the Cosmetics Rules, 2020, in triplicate.
- A signed and stamped specification sheet and product formula documents.
- Ownership document of the premises.
- Documents relating to the constitution of the applicant.
- The applicant might face certain roadblocks if he/she doesn't have following documents during the process:
- COA including physical and chemical parameters along with validated test methods.
- Ownership document, rental agreement.
- Blue print of the Premises and List of machineries and equipment along with their locations.
Expert Advise
CliniExperts provides the following advice to its clients-
The applicant must have a copy of the approved layout plan of the manufacturing area.
The applicant must ensure that there are no non-cosmetic claims mentioned on the label.
The applicant should confirm that the premise is not located in a residential area.
The applicant must ensure all the addresses, names, and other details mentioned on every document are the same.
The applicant should ensure all the documents are in line with the New Cosmetics rules 2020.
Why Choose CliniExperts?
By partnering with CliniExperts, manufacturers benefit from a streamlined, expert-led approach that minimizes the complexity of regulatory compliance, enhances the accuracy of your application, and accelerates your entry into the Indian market.

Expert Guidance
CliniExperts assist with the registration process with regulatory bodies and ensure compliance with all regulatory requirements, including the timely submission of necessary documents.

Thorough Document Review
CliniExperts conduct a meticulous review of all documents, perform a GAP Analysis to identify and address any deficiencies, and communicate any issues promptly to ensure a smooth application process.
Final Review and Fee Management
CliniExperts oversee the final review of your application and coordinate the payment of government fees, ensuring that all financial aspects are handled efficiently.

Timely Follow up
CliniExperts will maintain timely follow-ups with the relevant Government bodies to keep everything on track.
Regulatory Compliance
CliniExperts ensure that all submissions are in strict adherence to the regulatory guidelines.
Related Services
Sugam Registration for Medical Devices and ivd
Importer | Regulatory Body: CDSCO
The registration process includes several steps to obtain approvals. The process involves registration, undertaking, and uploading the documents, complying with the given rules and regulations. We at CliniExperts, make sure that our expert team creates your registration easy and quick so that you can focus on more important aspects of your product launch...
Test license to manufacture In Vitro Diagnostics in India – MD 12 & MD 13
Manufacturers | Regulatory Body: CDSCO
Registration for in-vitro diagnostic medical devices for manufacturing can be time-consuming and tedious; we at CliniExperts will take care of all the registration procedures while you focus energies on planning your product launch. From the application process forms to undertaking a license test, we assist in every registration aspect to help you smoothly start the manufacturing process.
Import License For In-Vitro Diagnostic Kits in India – Form MD14 & MD15
Importers | Regulatory Body: CDSCO
Get Your Import License for In-Vitro Diagnostic Kits (IVD) and Medical Devices with CliniExperts. Hassle-free assistance in providing Import License of Form MD 15.
Frequently Asked Questions
What are the labelling requirements needed to comply with manufacturing cosmetics in India?
The cosmetics label manufactured in India should comply with the provisions specified in rule 34 of the Cosmetics Rules, 2020.

What is the validity of the Cosmetic Manufacturing License?
According to rule 30 of the Cosmetics Rules 2020, the Cosmetic Manufacturing License will remain valid for five years.

Can cosmetics be tested on animals in the country?
No. According to rule 39(7)of the Cosmetics Rules, 2020, no person is allowed to use or test cosmetics on animals.

If homemade cosmetics require Manufacturing license from State FDA?
To obtain cosmetic manufacturing license, you must have a manufacturing facility located in industrial area.

