CDSCO Provides Guidelines For Approval Of COVID-19 Vaccines For Restricted Use In An Emergency Situation In India And For The Import Of Vaccine By The Private Sector

Approval of COVID-19 vaccines for restricted use in India
On the 15 th April, 2021, the National Expert Group on Vaccine Administration for the COVID-19 recommended the authorization for the emergency use of COVID-19 vaccine, which is already approved for restricted use by US FDA, EMA, UK MHRA, PMDA Japan, or listed in WHO Emergency Use Listing.
The DCGI will grant permission to the applicant for using vaccines for restricted use under the following conditions:
The vaccines used should follow the guidelines mentioned in the National COVID-19 vaccination programme. The first 100 individuals vaccinated should be monitored for safety for 7 days. Within 30 days of approval of the vaccine, the applicant should initiate post-approval bridging clinical trials.
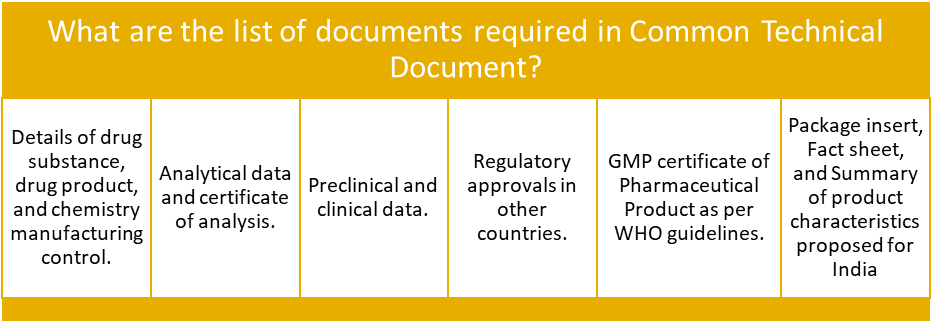
What is the procedure for processing the application?
- Foreign manufacturers can apply for approval of the COVID-19 vaccine via their Indian subsidiary or an authorized agent in India as per the rules under the Drugs and Cosmetics, Act, 1940.
- The application can be submitted via the SUGAM online portal along with the mandatory documents and fees as shown in table above. CDSCO will process these applications on the highest priority as per the requirement of New Drugs and Clinical Trial Rules, 2019 and DCGI and will decide within 3 working days.
- Additionally, the applicant may apply for an import registration certificate and import license as well as for permission for Restricted use in Emergency situation for smooth completion of the process.
- CDSCO will process the import registration certificate and import license within 3 working days post-approval of the permission for Restricted use in Emergency situation.
- After the approval of the registration certificate and import license, the applicants need to get batch release certificate for each and every batch from Central Drug Laboratory (CDL), Kasauli, before it can be used further.
- After obtaining an approval receipt from the CDL, the vaccines should be initially tested on only 100 individuals.
- The safety data sent by the applicant will be reviewed by CDSCO and further authorize the vaccine, if data found satisfactory.
- CDSCO will consult the Subject Expert Committee and approve the bridging trial protocol within 7 days after the proposal.
- Post-approval, the applicant needs to conduct the trials within the allocated timelines and submit the data to CDSCO.
- After receiving the data for bridging trials, the DCGI will assess the permission granted for the vaccines for restricted use.
- Post-approval of the restricted use, the proposed site for fill-finish within India, which is different from the manufacturing site, will be approved by the CDCSO based on the inspection and CDL release.
- The vaccine manufactured in India right from the drug substance stage to the fill-finish stage will also be granted license base on the inspection, stockpiling, CDL release.
Import of ready to use vaccines
The ready-to-use vaccines will be allowed to be fully utilized from 1 st May 2021 as per the guidelines on Liberalized Pricing and Accelerated National COVID-19 Vaccination Strategy published by the Ministry of Health and Family Welfare.
How can private sector or any government sector entity import the COVID-19 vaccine?
Any private or government entity who wishes to import Covid-19 vaccine for vaccination needs to follow the following process:
- For vaccines not yet approved or licensed in India, the agent of the manufacturer or the importer should take the permission/licenses mentioned in the table 1 from CDSCO.
- After receiving the licence, the authorized agent or importer can import the vaccine, and the private sector entity can obtain the vaccines as per the National guidelines.
- The vaccine which is already approved or licensed for import by the CDSCO, any entity can procure the vaccine from the licensee or the importer for its use as per the National Guidelines.

Summary
- The 23rd meeting of the National Expert Group on Vaccine Administration for COVID-19 held on 11 th April 2021 laid out the guidelines for the emergency use of vaccines.
- The ready to use vaccines will be allowed to be fully utilized from 1st May as per the guidelines published by the Ministry of Health and Family Welfare.
References
- Guidance for approval COVID-19 Vaccines in India for restricted use in emergency situation which are already approved for restricted use by UD FDA, EMA, UK MHRA, PMDA Japan or which are listed in WHO Emergency Use Listing (EUL). Available at: https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=NzE0Mw== . Accessed on May 14, 2021.
Recent Posts
Organic Food Labelling In India| Certification, and Import of Organic Food in India

This Article is All About Organic Food Labelling In India and Certification, and Import of Organic Food in India. Explained in Detail About What is Organic Food labelling? Summary Short Description Wi..
Cosmetic Label Compliance India : A Guide to Compliance

Introduction Looking for Cosmetic Label Compliance India? Are you a cosmetic manufacturer or importer navigating the complex world of Indian regulations? Ensuring your product labels comply with the l..
Clinical Investigation Approvals: An Overview of Forms MD-22 and MD-23

Summary Short Description Strict regulatory protocols govern clinical investigations for medical devices. Central to this process are forms MD-22 and MD-23. Form MD-22 is an application to Central Lic..
HAVE A QUERY?
REACH US!Office
New Delhi
Unit No. 324 & 325, City Centre Mall, Plot No. 5, Sector 12, Dwarka, India - 110075
+917672005050
Bengaluru
RMZ Galleria, 1st floor, Ambedkar Colony, Yelahanka, Bengaluru, Karnataka, India – 560064
Call us on
Sales: +91 7672005050
Reception: +91-11-45214546
Timings
9 am to 6 pm (Monday to Friday)


