Free Sale Certificate from CDSCO for regulated Medical Devices in India - FSC

A Free Sale Certificate for Medical Devices is mandatory to import or export of medical devices to and from India. CliniExperts helps medical device companies secure A Free Sale Certificate or Certificate for Export of Medical Devices.
Free Sale Certificate from CDSCO for regulated Medical Devices – Overview
A free sale certificate, also known as a "Certificate for Export" is a document issued by the national regulatory authority. The Central Drugs Standards Control Organization (CDSCO) streamlines the application process for free sale certificate for notified medical devices in India.
The manufacturer can obtain a Free Sale Certificate of regulated /notified medical device through online medical device portal and the certificate shall be issued by CDSCO within 30 working days. Application for the free sale certificate must be filled out online via the Sugam portal with a list of the necessary documents.
Who Can Apply?
Any manufacturer holding a valid manufacturing license can apply for the Free sale certificate from CDSCO.
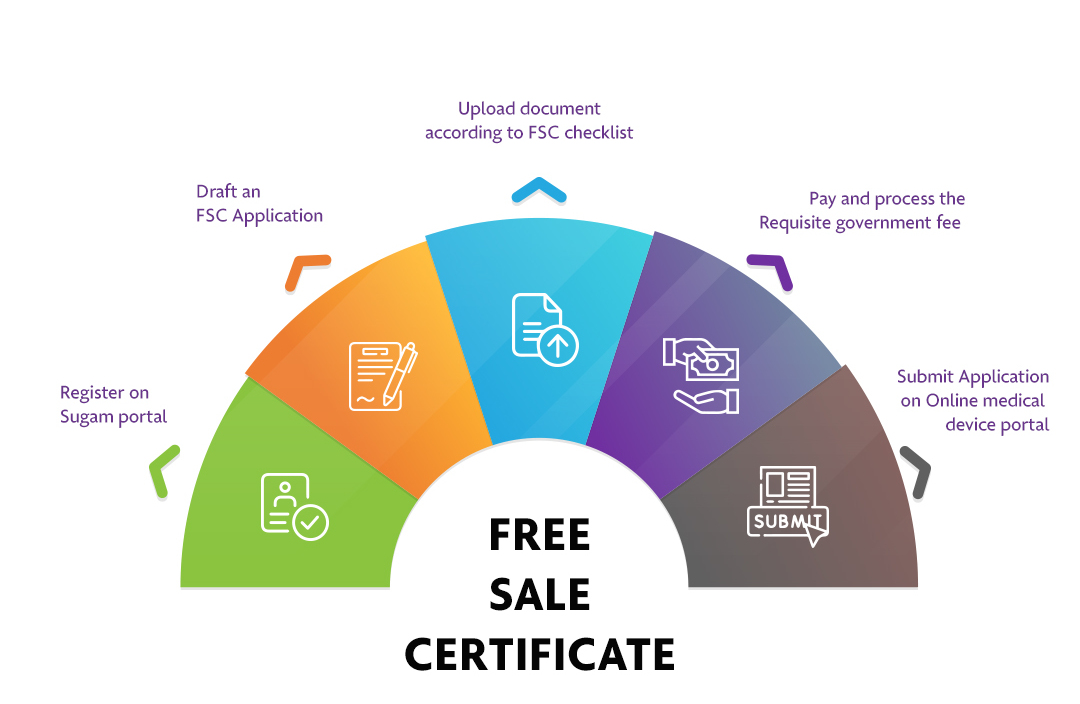
How To Apply?
The Applicant must follow the following process:
-

Step 1: Register on Sugam portal
-

Step 2: Draft an FSC Application
-

Step 3: Upload document according to FSC checklist
-

Step 4: Pay and process the Requisite government fee
-

Step 5: Submit Application on Online medical device portal

Validity
A Free Sale Certificate is valid for as long as the Manufacturing license is valid.

Fee Involved
INR 1000 for each separate medical device.Important Documents

- Cover letter on letterhead with the applicant's name and address, duly signed and stamped by the organization's head.
- A Rs.100 notarized stamp paper undertaking (Against No market complaint and adverse event)
- A word file containing a list of products for which a free sale is required.
- A manufacturing license as well as a list of approved products.
Timeline to get
FSC
from Central Drugs Standard Control Organisation
30
WORKING DAYSEssential Tips
- A valid manufacturing license is the most important requirement for obtaining FSC from CDSCO
- A Legal Undertaking on 100Rs stamp paper (Against No Market Complaint and Adverse Event) is a must.
- The applicable government fee must be paid.
- To qualify for FSC certification through CDSCO, products must fall under the notified categories
- A manufacturer with no complaints against their products can easily obtain the FSC from CDSCO.
Expert Advise
All the documents must comply with the FSC checklist.
Manufacturer must have a valid license.
Related Services
Permission for Loan License to Manufacture Class C & D Medical Devices in India
Importer | Regulatory Body: CDSCO
To increase the production of your existing notified medical devices can help you soar to new heights. Contact CliniExperts today to help you sail through the paperwork and obtain the Loan License for Manufacturing of Class C and Class D notified medical devices via Form MD – 8 and Form MD – 10 in no time!
Permission for Loan License to Manufacture Class A - B Medical Devices in India
Importer | Regulatory Body: SLA
Achieve the success that your medical business deserves. Permission for loan license to manufacture class A - B medical devices helps you to boost the production and attain new heights. CliniExperts guides you throughout the complete regulatory process to get the grant of loan license to manufacture medical devices in Form MD 6.
SUGAM Registration- MD/ IVD in India
Importer | Regulatory Body: CDSCO
The registration process includes several steps to obtain approvals. The process involves registration, undertaking, and uploading the documents, complying with the given rules and regulations. We at CliniExperts, make sure that our expert team creates your registration easy and quick so that you can focus on more important aspects of your product launch.
Permission to Manufacture Class B Medical Devices in India
Importer | Regulatory Body: CDSCO
The application for manufacturing, sale or distribution of any medical device includes a tedious list of processes and forms CliniExperts team consists of experts working in the same domain with practical experience and knowledge of the governing rules. Hence, we provide a one-stop solution for all the licensing and approval needs of your firm.
Import License for Medical Devices in India – Form MD 14 & MD 15
Importer | Regulatory Body: CDSCO
Meet all your Regulatory Compliance needs. CliniExperts' professionals help you plan and streamline regulatory approval processes.
Frequently Asked Questions
Is the FSC application process currently conducted online or in person?
The FSC application process has been made available online by CDSCO.

What is the FSC application fee?
Each medical equipment requires a fee of INR 1000.

Can the CDSCO provide FSC for non-notified products?
No

