Test license to manufacture In Vitro Diagnostics in India - MD 12 & MD 13

Registration for in-vitro diagnostic medical devices for manufacturing can be time-consuming and tedious; we at CliniExperts will take care of all the registration procedures while you focus energies on planning your product launch. From the application process forms to undertaking a license test, we assist in every registration aspect to help you smoothly start the manufacturing process.
Test license to manufacture In Vitro Diagnostic Kits (Form MD 12, 13) – Overview
A medical device under Class A, B, C, or D can be manufactured in small quantities for various purposes. For instance, clinical investigation, testing, evaluation, examination, demonstration, or training. For manufacturing any medical device, a particular set of procedures has to be followed, which is laid down by the CDSCO. The manufacturer has to apply to the Central Licensing Authority for approval. This is regarding the guidelines of CDSCO. The Central Licensing Authority maintains the quality and standards for medical device manufacturing. It serves as the highest regulatory body in the country. Any manufacturer wishing to produce a medical device must apply to the Central Licensing Authority. This is done by submitting Form MD-12. The form states the purpose for manufacturing a medical device. This purpose can be for testing, evaluation, demonstration, or training. The test license will be given in Form MD-13 by the online portal of the Ministry of Health and Family Welfare. The procedure duration can take up to 30 days for obtaining a test license.
Who Can Apply?
Any person who wishes to manufacture small quantities of medical devices concerning the in-vitro diagnostic medical devices can apply to the Central Licensing Authority. The applicant has to state the purpose of manufacturing medical devices.
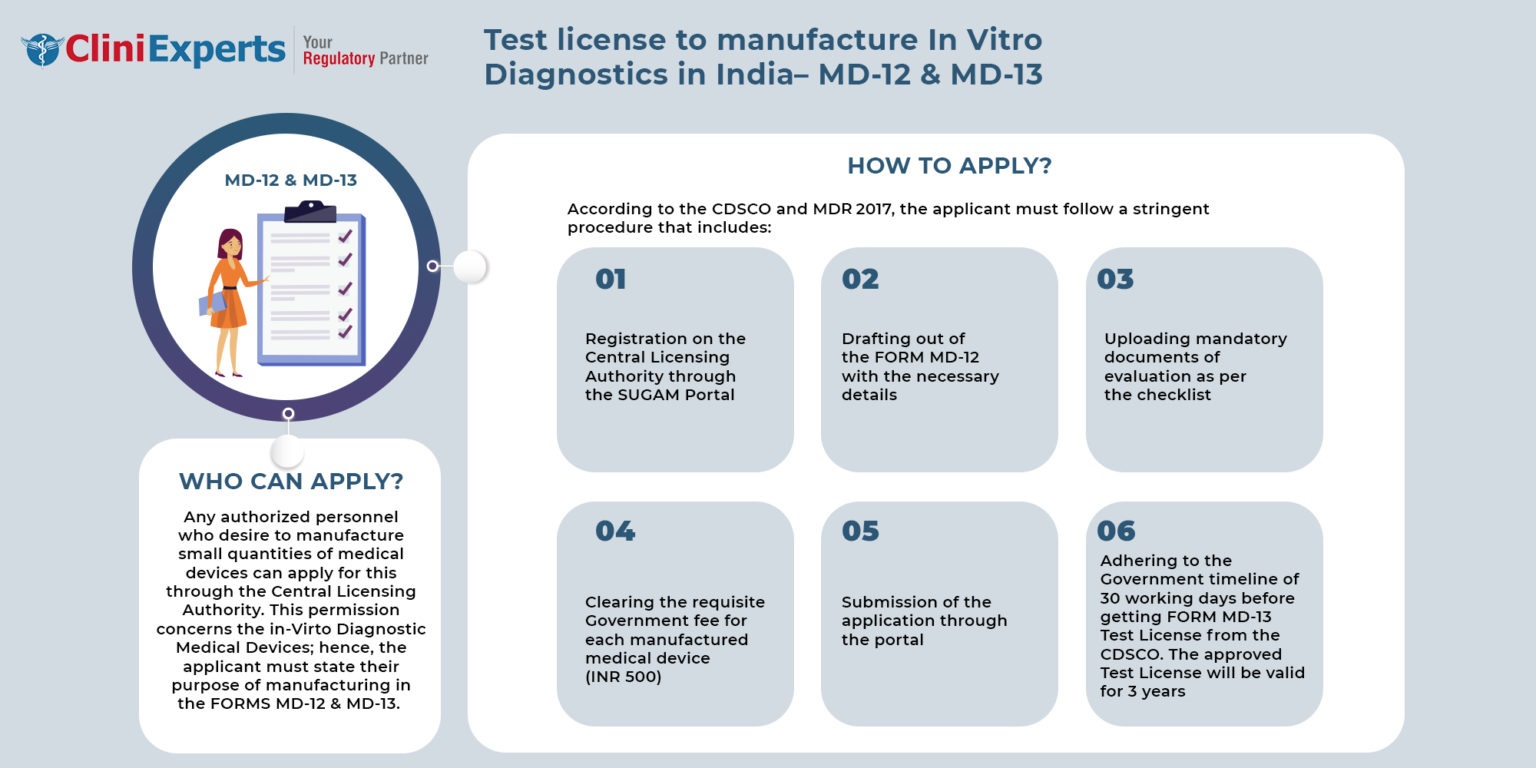
How To Apply?
The Applicant must follow the following process:
-

The applicant must apply to the Central Licensing Authority by registering on the SUGAM portal.
-

The applicant must apply with Form MD-12 with the required fees for each medical device.

Validity
Validity for test license to manufacture is 3 Years

Fee Involved
INR 500 for each distinct medical device.
Important Documents

The set of documents required for application of license are:
- A brief description of the In-vitro diagnostic kit which consists of
- The intended use
- The material used for construction
- The design – Its labels and IFU
Timeline to get
MD 13
from Central Drugs Standard Control Organisation
30
DAYSEssential Tips
- The documentation of every manufacturing unit should be well recorded. It should include the manufacturer’s name, specified quantity, and date of the manufactured device. This documentation should be kept with the holder of the test license.
- For every consignment of medical devices, an invoice or statement should be generated. It should include the name and quantity of the manufactured medical device.
- The approved test license should be used specifically. It should be used for clinical investigation, testing, evaluation, examination, demonstration, or training.
Expert Advise
The holder of the test license shall maintain record of the activities undertaken including the name of manufacturer, quantity manufactured and date of manufacture
The consignment of medical device shall be accompanied by an invoice or statement showing the name and quantity of medical device.
The Test license must be exclusively used for the purpose of clinical investigations, test, evaluation, examination, demonstration or training
Frequently Asked Questions
What is a Test License?
The Test License is the approved license given by the Central Licensing Authority for manufacturing or importing small quantities of in-vitro diagnostic medical devices for clinical investigation, test, evaluation, demonstration, or training purposes

Can we mention the multiple manufacturing sites in a single test license application?
Yes, you can mention multiple manufacturing sites by a single test license application intended for clinical investigation, testing, evaluation, demonstration, or training.

What is the procedure if one wishes to move a medical device from one location to any other site which has not been specified in the Test License?
In such scenarios, the applicant shall inform the Central Licensing Authority before relocating the medical device.

Within how many days can the suspended licensee appeal to the authority for cancellation of suspension?
After the suspension of the test license, the manufacturer/licensee can appeal to the authority within forty-five days from the date of a suspension order.

