Test License to Import Drugs in India - Form 12, CT-16 & Form 11, CT-17

Need permission to import small quantities of samples for the purpose of testing and analysis? CliniExperts' professionals know the latest laws and regulations related to the development, testing, manufacturing, approval, and commercialization of products and will provide complete guidance in securing a test license for importers or manufacturers looking to apply for Form 11 and CT-17.
Test License for Drug Importers – Overview
An importer or manufacturer who wants to import small quantities of samples for the purpose of testing and analysis must apply for a test license. The regulatory body involved in this process is the Zonal Food and Drug Administration (FDA). To apply for the test license, the applicant must visit the SUGAM portal and fill out the application in Form 12 or Form CT-16. During this process, the applicant must also submit all the necessary documents and pay the mandatory government fee.
Who Can Apply?
Importers and manufacturers both can apply for a test license for import.
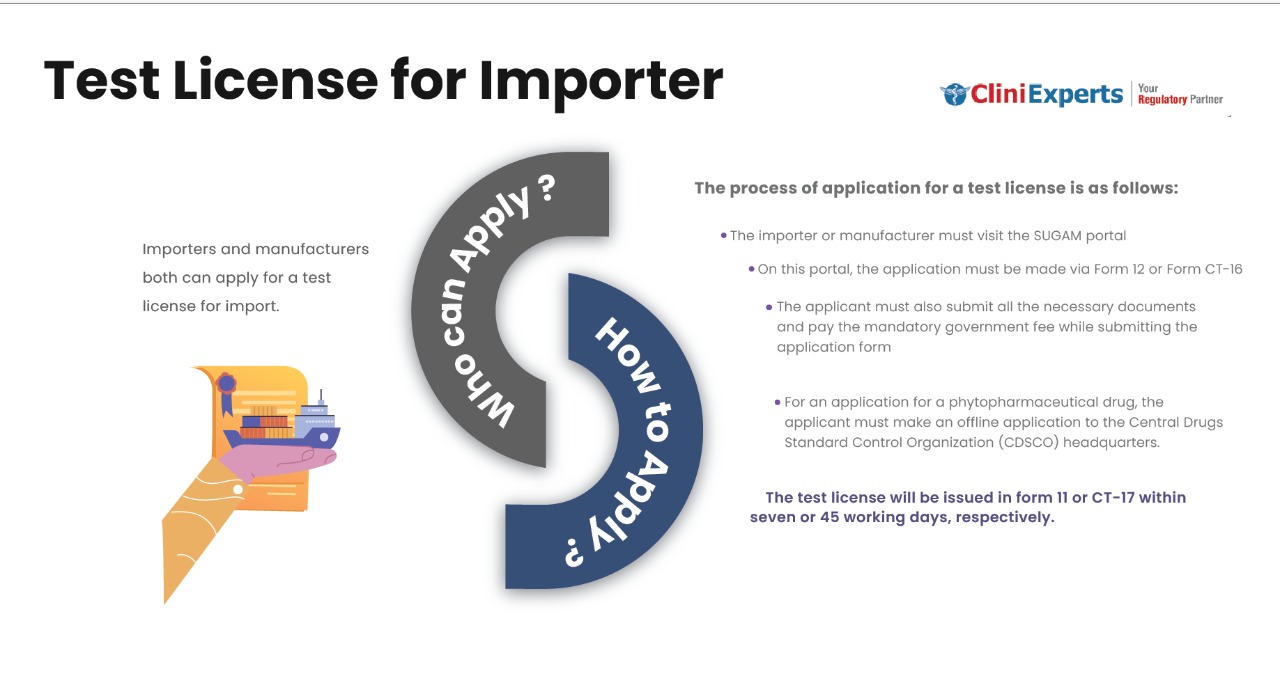
How To Apply?
The Applicant must follow the following process:
-

The importer or manufacturer must visit the SUGAM portal.
-

On this portal, the application must be made via Form 12 or Form CT-16.
-

The applicant must also submit all the necessary documents. They need to pay the mandatory government fee while submitting the application form.
-

For a phytopharmaceutical drug application, the applicant must apply offline. The application should be sent to the Central Drugs Standard Control Organization (CDSCO) headquarters. The test license will be issued in form 11 or CT-17 within seven or 45 working days, respectively.

Validity
The test license for the importer would be valid for three years from the date of issue.

Fee Involved
While applying for the test license via Form 12 or Form CT-16, the applicant must pay the required government fees.- Form 12: The applicant must pay INR 5000 for the first drug and INR 2000 for subsequent drugs in the same application
- Form CT-16: The applicant must pay INR 5000 per drug.
Important Documents

While making an online application for obtaining a test license for import, the applicant must upload the following documents:
- A copy of the manufacturing license or wholesale license in Form 20B/21B
- Manufacturing license in Form 25/28 along with product permission or a copy of DSIR certificate
- A justification of quantity that needs to be imported
- An undertaking for product under the Narcotic Drugs and Psychotropic Substances (NDPS) Act, 1985
- An undertaking for the samples not imported last three years
Timeline to get
Form 11, CT-17
is 7 Working Days and Timeline for CT-17 is 45 Workings Days from Central Drugs Standard Control Organisation
Essential Tips
- The documents required for the application process need to be signed and stamped by an authorized signatory
-
The applicant must adequately justify the quantity of drugs to be imported. Improper justification of quantity provided can delay the application process.
- The applicant must mention the quantity of samples to be used and the required test parameters of the tests to be performed
- To submit the application, it is essential for the company to have an active Sugam portal account
Expert Advise
The application forms must be signed and stamped by an authorized signatory
The applicant must ensure that the justification provided for the quantity of samples to be imported is clear with the testing parameters
Related Services
SUGAM Registration- MD/ IVD
Importer | Regulatory Body: CDSCO
The registration process includes several steps to obtain approvals. The process involves registration, undertaking, and uploading the documents, complying with the given rules and regulations. We at CliniExperts, make sure that our expert team creates your registration easy and quick so that you can focus on more important aspects of your product launch...
Test license to manufacture In Vitro Diagnostics in India – MD 12 & MD 13
Manufacturers | Regulatory Body: CDSCO
Registration for in-vitro diagnostic medical devices for manufacturing can be time-consuming and tedious; we at CliniExperts will take care of all the registration procedures while you focus energies on planning your product launch. From the application process forms to undertaking a license test, we assist in every registration aspect to help you smoothly start the manufacturing process.
Import License For In-Vitro Diagnostic Kits in India – Form MD14 & MD15
Importers | Regulatory Body: CDSCO
Get Your Import License for In-Vitro Diagnostic Kits (IVD) and Medical Devices with CliniExperts. Hassle-free assistance in providing Import License of Form MD 15.
Frequently Asked Questions
Why is the purpose for issuing a test license in Form 11 or CT-17?
An importer or manufacturer can use a test license issued in Form 11 or CT-17 to import small quantities of samples for the purpose of testing and analysis

Where can the applicant apply for a test license?
The applicant must visit the SUGAM portal and fill out the application in Form 12 or Form CT-16.

What are the mandatory government fees during the application process for a test license?
While applying for the test license via Form 12, the applicant must pay INR 5000 for the first drug and INR 2000 for subsequent drugs in the same application. Similarly, while making an application via Form CT-16, the applicant must pay INR 5000 per drug.

What documents are necessary to be submitted during the application process?
While making an online application for obtaining a test license for import, the applicant must upload the following documents: o A copy of the manufacturing license or wholesale license in Form 20B/21B o Manufacturing license in Form 25/28 along with product permission or a copy of DSIR certificate o A justification of quantity that needs to be imported o An undertaking for product under the Narcotic Drugs and Psychotropic Substances (NDPS) Act, 1985 o An undertaking for the samples not imported last three years

What is the timeline for obtaining a test license?
The timeline for obtaining a test license under Form 11 is seven working days, and Form CT-17 is 45 days.

