Approval of Non Specified Ingredients/ Products - For Food Supplements & Nutraceuticals in India

CliniExperts is backed by highly skilled professionals. Our experts are updated with the latest FSSAI laws and guidelines. They assist in easy sailing through the FSSAI license application process with minimal paperwork. They will guide you to register and get approval for non-specified food supplements ingredient/products in India.
Approval of Non Specified Ingredients/ Products - For Food Supplements & Nutraceuticals – Overview
The food supplement sector is growing faster due to multiple innovations compared to earlier times. Nowadays, new molecules, substances and botanicals are included in food supplements. With globalization, inter country trade is regular which helps to access novel products into Indian market. However, these novel products may not be listed under FSSAI regulation.
As a result, getting such products registered and approved per FSSAI regulations is essential. Before importing or manufacturing such products, they must be registered under Non-Specified Foods. The regulatory body that governs approvals for all food products in India is the Food Safety and Standard Authority of India (FSSAI). The aim is to provide approvals of Non-Specified Foods to importers or manufacturers before launching it into the market. The approval process could take six to twelve months.
Who Can Apply?
Any Indian based manufacturer, importer or marketer involved in NSF businesses can apply for this service.
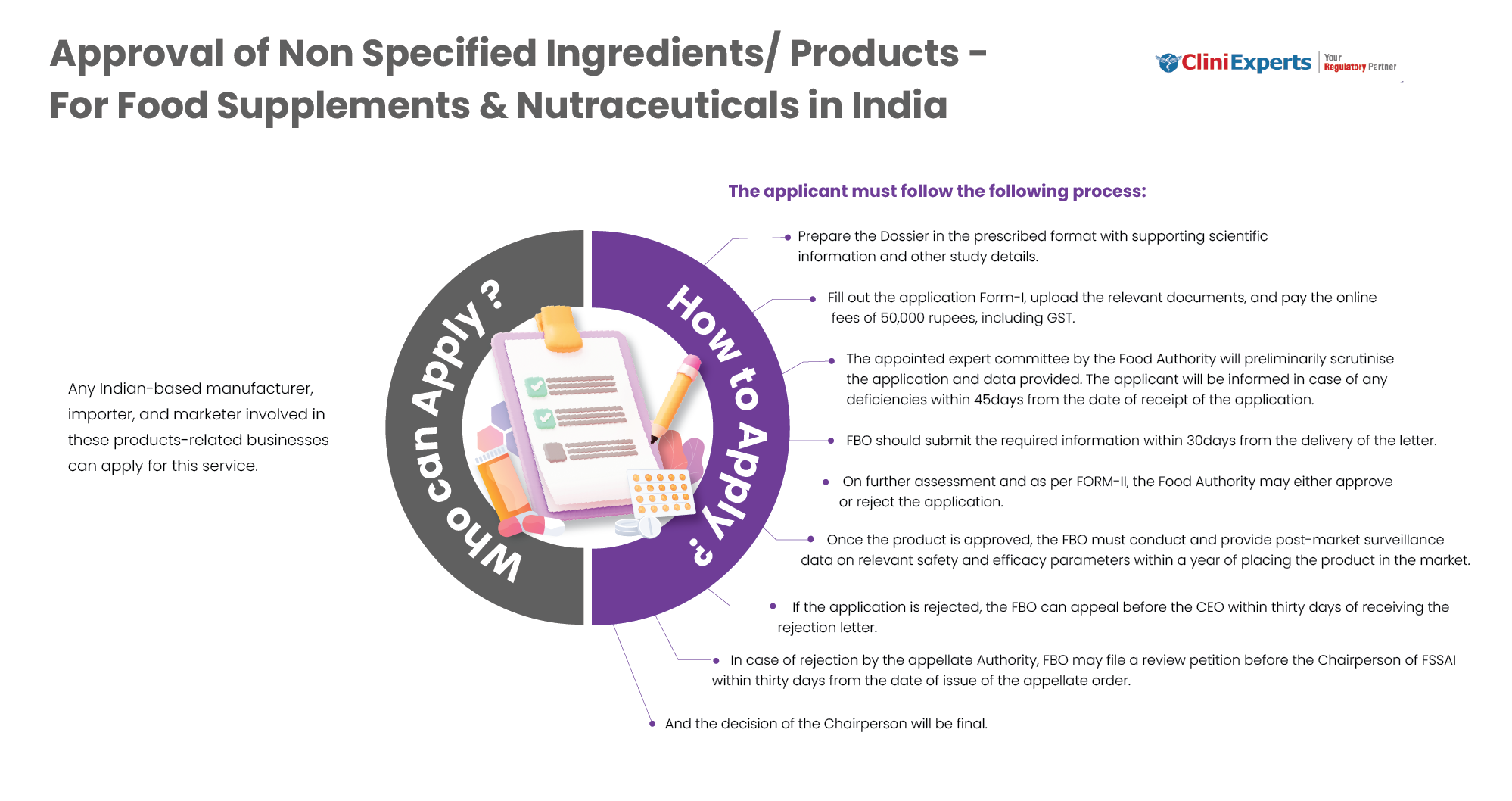
How To Apply?
The Applicant must follow the following process:
-

Prepare the Dossier in the prescribed format with scientific information and other study details.
-

Fill out the application Form-I, upload the relevant documents, and pay the online fees of 50,000 rupees, including GST.
-

The appointed expert committee by the Food Authority will preliminarily scrutinize the application. The applicant will be informed in case of any deficiencies within 45 days from the date of receipt of the application.
-

FBO should submit the required information within 30 days from the delivery of the letter.
-

On further assessment and as per FORM-II, the Food Authority may either approve or reject the application.
-

Once the product is approved, the FBO must conduct post-market surveillance. They need to provide data on relevant safety and efficacy parameters within a year of placing the product in the market.
-

If the application is rejected, the FBO can appeal before the CEO within thirty days of receiving the rejection letter.
-

If the appellate Authority rejects the case, the FBO can appeal to the Chairperson of FSSAI within thirty days from the date of the appellate order.
-

And the decision of the Chairperson will be final.

Validity
The approval of Non-Specified Food Supplements and Nutraceuticals under FSSAI regulation has no fixed maturity. Instead, the License is valid for a lifetime unless the Government changes the said regulation.

Fee Involved
The Government charges a pre-requisite fee of 50000 rupees + GST to register products/ingredients under Non-Specified Foods in India.Important Documents

The essential documents involved are:
- Product-related information.
- Source of food ingredients and details of the species.
- Manufacturing process with description and flow chart.
- If available, a copy of the License number
- Functional use details
- Intended use details
- Worldwide Regulatory Status.
- A lab test certificate of analysis from NABL or ILAC recognized laboratory.
- Safety and allergenicity details
- Copy of agreement of relationship between applicant and manufacturer and other entities involved.
- Supporting claim statements with clinical trials or peer-reviewed journals for substantiation.
- Safety in sensitive groups like pregnant women, lactating mothers, children or any special group.
- Copy of the proposed product prototype label as per relevant FSS Regulations.
- Declaration to conduct and provide post-marketing surveillance data if indicated in Form II.
- Submitting additional information applicable to the specific category and the general information.
Timeline to get from Food Safety and Standards Authority
6 to 12
MONTHSEssential Tips
The following tips may benefit the applicants during the application process:
- It is mandatory to submit COA from the third-party NABL/ILAC-recognized lab.
- FBOs should mention the end-use of the ingredient/pre-mix/product.
- It is recommended to provide the serving size of the product (no. of serving/day). The following roadblocks may be faced during the application process. Thus, here are tips to ensure smooth processing of the application.
- FBOs need to specify end-use based on the usage level, target group, duration of use etc.
- COA should include physical and chemical parameters of the product, along with validated test methods.
- Technical specification document of product must have tests/specifications with tolerance limit. If not, then providing a straightforward method is necessary.
Expert Advise
CliniExperts provides the following advise to its clients-
Applicant should declare the details of the parties involved in selling the NSF product in the Dossier. This is mandatory to increase the traceability of the product.
Applicant must look for appropriate product categorization to receive approval. Otherwise, the application might be rejected.
Randomized clinical trial studies are essential for substantiating safety and functional use details. Peer-reviewed journals with high impact factors must also be utilized in this regard.
Related Services
SUGAM Registration- MD/ IVD
Importer | Regulatory Body: CDSCO
The registration process includes several steps to obtain approvals. The process involves registration, undertaking, and uploading the documents, complying with the given rules and regulations. We at CliniExperts, make sure that our expert team creates your registration easy and quick so that you can focus on more important aspects of your product launch...
Test license to manufacture In Vitro Diagnostics in India – MD 12 & MD 13
Manufacturers | Regulatory Body: CDSCO
Registration for in-vitro diagnostic medical devices for manufacturing can be time-consuming and tedious; we at CliniExperts will take care of all the registration procedures while you focus energies on planning your product launch. From the application process forms to undertaking a license test, we assist in every registration aspect to help you smoothly start the manufacturing process.
Import License For In-Vitro Diagnostic Kits in India – Form MD14 & MD15
Importers | Regulatory Body: CDSCO
Get Your Import License for In-Vitro Diagnostic Kits (IVD) and Medical Devices with CliniExperts. Hassle-free assistance in providing Import License of Form MD 15.
