CDSCO
In-Vitro Diagnostics Manufacturing Services
CliniExperts served 50+ IVD manufacturers so far. We manage all regulatory and compliance mandates seamlessly
Choose CliniExperts for your regulatory needs. Our proven leadership, ethics, and efficiency have earned us a roster of 50+ satisfied clients, including industry giants. We deliver zero-defect filings and provide a delightful end-to-end experience. Trust us to be your preferred regulatory partner and take advantage of our full-stack services.
CDSCO – How it governs medical device regulatory affairs
The CDSCO is responsible for regulating the manufacture, import, and sale of IVD devices in India. It has established a regulatory framework that requires IVD manufacturers to comply with various requirements related to registration, classification, clinical performance evaluation, labeling, adverse event reporting, and post-market surveillance. Manufacturers must follow the regulations to ensure that their products are safe and effective for use in India. The CDSCO's role is to enforce the regulations, monitor the quality and safety of IVD devices, and take appropriate action in case of any non-compliance. By ensuring that IVD devices are safe and effective, the CDSCO helps to protect the health of the people of India.
Trust CliniExperts to handle your regulatory process from start to finish with expert experience and a zero-defect approach

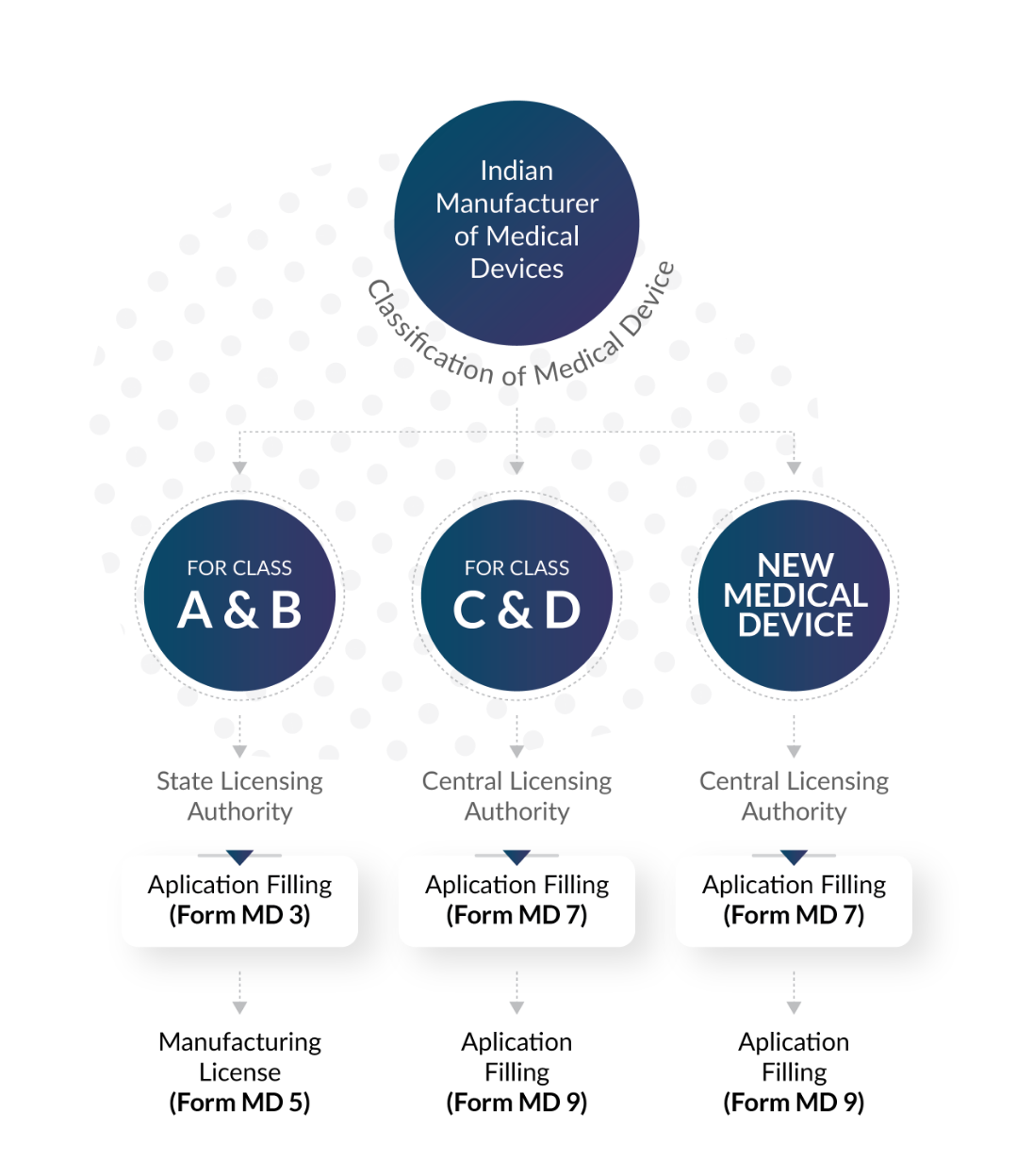

ABOUT CLASS A, B, C and D In-vitro Diagnostics
As per the New Medical Rules, 2017, all IVD’s have been classified into four distinct classes. According to the MDR, the categories are Class A, Class B and Class C, and Class D. This the range of medical devices governed by CDSCO:
Class A IVD devices are considered to have low risk and are usually exempt from regulatory control. Examples of Class A IVD devices include reagents and solutions used for laboratory testing.
Class B IVD devices are considered to have moderate risk and may require limited regulation. Examples of Class B IVD devices include pregnancy test kits, blood glucose test strips, and urine analysis test strips.
Class C IVD devices are considered to have high risk and require more extensive regulation. Examples of Class C IVD devices include HIV testing kits, hepatitis testing kits, and cancer diagnostic kits.
Class D IVD devices are considered to have the highest risk and require the most extensive regulation. Examples of Class D IVD devices include genetic testing kits, companion diagnostics, and certain laboratory-developed tests.

Class A and B In-vitro Diagnostics
An in-vitro diagnostic (IVD) is a product used for disease diagnosis, treatment, or prevention in humans or animals. IVDs are classified into four risk-based classes: Class A, B, C, and D. Class A and B IVDs are considered low-to-moderate risk. Class A IVDs are low-risk devices that require little invasiveness, such as specimen receptacles and laboratory instruments. Class B IVDs are low-to-moderate risk devices with minimal invasiveness, such as fertility and cholesterol tests
Class C and D In-vitro Diagnostics
IVD devices are classified into four risk-based classes: Class A, B, C, and D. Class C and D IVDs are considered moderate high risk and high risk, respectively. Class C IVDs detect infectious agents with the potential to cause severe disability or death. Class D IVDs detect life-threatening or highly contagious infectious diseases. To manufacture and distribute Class C or Class D IVDs, companies must obtain authorization using Form MD-9, and a manufacturing license can be obtained using Form MD-7.
New In-vitro Diagnostics
New in-vitro diagnostic devices (IVDs) are those that have not been previously marketed in India, or have undergone substantial modifications that may affect their safety or performance. The Central Drugs Standard Control Organization (CDSCO) is the regulatory authority in India responsible for reviewing and approving new IVDs for marketing and distribution. The review process for new IVDs typically includes an evaluation of the technical documentation, clinical performance data, and labeling to ensure that the device is safe and effective for its intended use.
However, obtaining the required licences, certifications, and approvals for these devices is a herculean undertaking. More than 40 specific documents, covering 12 different factors, must be filed just for the plant master file. Additionally, there is the equally lengthy Device Master File, SUGAM portal registration, ISO 13485 certification, Clinical Performance Evaluation, and a seemingly infinite list of other regulatory requirements.
That’s not all. A new licensing regime for these devices comes into effect from 01-10-2023.
Existing Devices
| Applicant | Risk/Class | Type of Licence | Forms |
| Importer | A,B,C,D | Importer Licence | Application: MD-14, Application: MD-15 |
| Manufacturer | A,B | Manufacturing Licence | Application: MD-3, Application: MD-5 |
| Loan Licence | Application: MD-4, Application: MD-6 | ||
| C,D | Manufacturing Licence | Application: MD-7, Application: MD-9 | |
| Loan Licence | Application: MD-8, Application: MD-10 |
New Devices
| Applicant | Risk/Class | Type of Licence | Forms |
| Importer | A,B,C&D | Permission to conduct Clinical Performance Evaluation | Application: MD-24, Application: MD-25 |
| A,B,C&D | Import Licence | Application: MD-26, Application: MD-27 | |
| A,B,C&D | Test Licence | Application: MD-16, Application: MD-17 | |
| Manufacturer | A,B,C&D | Clinical Investigation Permission | Application: MD-22, Application: MD-23 |
| A,B,C&D | Manufacturing Licence | Application: MD-26, Application: MD-27 | |
| A,B,C&D | Test Licence | Application: MD-16, Application: MD-17 |
How It Is Regulated?
- IVDs are classified into four classes based on their risk: Class A, Class B, Class C, and Class D.
- To manufacture and sell IVD devices in India, companies need to obtain a manufacturing license from the CDSCO.
- The CDSCO requires that the manufacturer provide technical documentation, clinical performance data, and labeling for the IVD device.
- IVD manufacturers in India must also comply with various other regulations and guidelines issued by the CDSCO, such as the Indian Pharmacopoeia, the Medical Device Rules, and the Good Manufacturing Practices guidelines for medical devices.
- The CDSCO conducts inspections to ensure that manufacturers are complying with these regulations and guidelines.

CliniExperts Can Assist In
At CliniExperts, we have become the preferred choice for regulatory partnerships in the medical device industry. Here's why:
- Strong client base and high retention rate. With a growing client roster and 100% retention rate, we've established a prominent market stature and size.
- Emphasis on ethics, integrity, and excellence. We are a responsible and respected organization with the necessary resources to navigate regulatory complexities.
- Talented and committed teams. Our teams, led by industry experts, are fully committed to delivering high-quality services.
- Up-to-date knowledge and information. We pride ourselves on maintaining cutting-edge knowledge and information to ensure that our applications are always compliant.
Together, these features have earned us a reputation as the most favored regulatory service provider. Let's collaborate to form a mutually beneficial and enduring partnership!
Form Names: MD3, MD5
Permission to Manufacture Class A & B In Vitro Diagnostics
The licensing authority in India is the Central Standard Control Organisation (CDSCO). The licensing process in a nutshell: Fill up the exhaustive application Form MD-3 and upload it on the SUGAM portal State licensing authorities issue permission to manufacture Class A and B In-vitro diagnostics on Form MD-5 after a grueling evaluation process
Regulatory Body Requirement
The purpose of this service is to obtain license to manufacture Class A and B IVD's in India. The State Licensing Authority is the regulatory body associated with this service. It grants the license to manufacture IVDs for sale or distribution in FORM MD-5 as per Medical Device Rule 2017.
Any person or manufacturing company wanting to manufacture a Class A or Class B IVDs must follow a certain procedure. The applicant must make an application to the State Licensing Authority (SLA) through the identified online portal of the Ministry of Health and Family Welfare in the Central Government in FORM MD-3.
How to Apply
The manufacturer must apply for the Grant of License to manufacture for sale and distribution of Class A or Class B Medical Devices in FORM MD-3 to the SLA.
The following documents need to be submitted along with the application:
- A cover letter
- Constitution detail of the firm, the Establishment/Site ownership/Tenancy Agreement
- A copy of duly notarised valid copies of the Quality Certificate concerning the manufacturing site,
- Plant Master file, Device Master file, Performance Evaluation Report (IVDs only)
- Test License obtained for testing and generation of quality control data
- Undertaking signed mentioning the manufacturing site complies with the provision of the Fifth schedule.
The State Licensing Authority reviews the application and appoints a Notified body to conduct an audit of the manufacturing site for Class B Medical Devices. Upon successful audit, the SLA issues the manufacturing license in FORM MD-5.
Form Names: MD7, MD9
Loan License to Manufacture Class A & B In- Vitro Diagnostics
Financial constraints can prevent you from achieving the success that your business deserves. A loan for manufacturing new notified medical devices of Class A and Class B, or for increasing the scale of production of your existing notified medical devices can help you soar to new heights. Contact CliniExperts today to help you sail through the paperwork and obtain the Loan License for Manufacturing of Class A and Class B notified medical devices via Form MD – 4 and Form MD – 6 in no time!
Regulatory Body Requirement
For manufacturing an in-vitro medical device of Class A & B, a loan license must be taken before starting the manufacturing process at a site of another licencee. Any manufacturer who intends to produce a device can apply to the concerned State Licensing Authority. A manufacturer has to go through an online application process for the medical device under the standards laid down by the CDSCO. The application process involves filling the Form MD-4 as an application on the online portal of the Ministry of Health and Family Welfare.
After evaluating the data, the State Licensing Authority can accept/reject the submitted proposal. The acceptance of the Loan Manufacturing License will be given by Form MD-6. The overall process takes about two to three months after applying.
How to Apply
The application must be made under Form MD-4 to the State Licensing Authority. The form has to be submitted on the SUGAM online portal with the necessary documents and payment of the fees.
Form Names: N/A
In-vitro Diagnostics ISO 13485 Certification Assistance
From design, to R&D, testing, manufacturing, sales and distribution, storage, and end-usage – Medical device lifecycles are increasingly controlled by regulatory and compliance mandates. ISO-13485 is designed to ensure the safety, effectiveness, and accuracy of these devices at the user end. Hence, being ISO-13485 certified is critical for all stakeholders – Be it the designers, manufacturers, suppliers, service providers, or distributors.
Regulatory Body Requirement
The purpose of this service is to assist in ISO 13485 certification process. ISO 13485 Certificate is an important Certification required by the manufacturers, designers, suppliers, distributors, and service providers of all medical devices including in vitro devices (IVDs).
The regulatory body involved in the process is the Accredited Notified Body. The following procedure needs to be followed to avail the ISO 13485 certificate:
- Applicant needs to establish Quality Management as per ISO 13485 standards and implement them in the organization
- Upon implementation applicants can reach out to the Accredited Notified Body for an audit of the organization and certification.
- The timeline for the process is 6 to 9 months.
How to Apply
The following steps need to be followed for the application process:
- Step 1: Applicant must define the scope of the ISO 13485 Certificate, which will mention the objectives and scope of the certificate.
- Step 2: Applicant must select the notified body
- Step 3: Applicant must establish a quality management system
- Step 4: Applicant must work according to the established quality management system
- Step 5: Prepare for the audit
- Step 6: Get audited by the Accredited Notified Body.
The notified body will audit the company for two to ten days, depending on the size of the organisation. The organisation will obtain the certificate(s) if it passes the audit.
Form Names: MD-24, 25
Permission to conduct Clinical Performance Evaluation
Permission to conduct Clinical Performance Evaluation issued to manufacturer/importer by the Central Licensing authority who wants to conduct Clinical Performance Evaluation of a new IVD's.
Regulatory Body Requirement
The Central Licensing Authority, CDSCO, is the regulatory body associated with this service.
This service aims to obtain Permission to conduct a Clinical Performance Evaluation of all new in-vitro diagnostics (IVDs).
A manufacturer or an importer who intends to conduct a Clinical Performance Evaluation of a new In-vitro diagnostic can apply through this service.
- The applicant needs to make an application to the Central Licensing Authority.
- The application can be filed in FORM MD-24 through an identified online Medical Device portal of the Ministry of Health and Family Welfare.
- The Permission to conduct a Clinical Performance Evaluation will be obtained in FORM MD-25.
- The approval is a Government process and could take approximately 1 to 2 months.
How to Apply
Any manufacturer or importer who wants to conduct a Clinical Performance Evaluation of new IVD's can apply through the following procedure:
- The application for the Grant of Permission to conduct a Clinical Performance Evaluation of a new IVD can be made in Form MD-24.
- The application should be addressed to the Central Licensing Authority, CDSCO.
- The application should be submitted along with the required documents and the specified fees on the online SUGAM portal.
- The reference regulation for this service is the Medical Device Rules, 2017.
Form Names: MD7, MD9
Permission to Manufacture Class C & D In Vitro Diagnostics
The Central Drugs Standard Control Organization is the principal Indian authority in charge of overseeing the production and distribution of approved medical equipment (CDCSO). The regulatory authority in India is in charge of issuing permits and authorising the manufacture and use of medical equipment.
This agency uses FORM MD-7 and FORM MD-9 to grant manufacturing licences in compliance with the Medical Device Rules of 2017.
Regulatory Body Requirement
For manufacturing an in-vitro diagnostic medical device (IVD) of Class C & D, a loan license must be taken before starting the manufacturing process. Any manufacturer who intends to produce a device at a site where the same device is being produced by another manufacturer must get a loan license. The person wanting to manufacture can apply to the Central Licensing Authority. A manufacturer has to go through an online application process for the medical device under the standards laid down by the CDSCO on the SUGAM portal. The application process involves filling the Form MD-7 as an application on the online portal of the Ministry of Health and Family Welfare.
How to Apply
A manufacturer must apply for a Grant of License to manufacture for Sale and distribution Class C or Class D medical devices
- The application must be made under Form MD-7 to the Central Licensing Authority. The application must be filled on the SUGAM portal by uploading the listed documents and paying the stated fees.
- After the registration, make sure you apply for the Test License Form MD-13 and performance evaluation test.
Form Names: MD8, MD10
Loan License to Manufacture Class C & D In- Vitro Diagnostics
Financial restraints might keep your company from obtaining the success it deserves. A loan for the production of new Class C and Class D notified In-vitro Diagnostics, or for boosting the scale of production of your current Class C and Class D notified In-vitro diagnostics, will help you fly to new heights.
Contact CliniExperts immediately for assistance in navigating the paperwork and quickly obtaining the Loan License for Manufacturing of Class C and Class D notified In-vitro diagnostics through Form MD - 8 and Form MD - 10!
Regulatory Body Requirement
According to the Medical Devices Rules, 2017, a loan manufacturing license in Form MD-10 is required for loan manufacturing of Class C & D (Notified) medical devices in India. These loan license applications are filed in Form MD-8, which is used to obtain the licenses in Form MD-10.
In order to obtain a loan license to manufacture Class C & Class D In-vitro diagnostics, the manufacturer has to make an application to the Central Licensing Authority via an online portal of the Central Government. It takes between 4-5 months to complete the process.
How to Apply
- Step 1: Sugam Registration of the applicant
- Step 2: Drafting the Application
- Step 3: Uploading all the documents according to checklist of the form MD-8
- Step 4: Processing of the mandatory government fee
- Step 5: Submitting the application on online Medical Device portal
Form Names: N/A
SUGAM Registration- MD/ IVD
The Ministry of Health and Family Welfare established the versatile and efficient SUGAM portal as part of the ambitious Digital India initiative. The SUGAM interface, with its 3600 tracking, querying, upload/download, and many other useful features, facilitates digital processing of almost all requirements related to Class A and B in-vitro diagnostics.
CliniExperts' extensive knowledge of this portal can help to accelerate these processes in a ZERO-ERROR digital environment.
Regulatory Body Requirement
The registration process for medical devices is primarily done on the SUGAM portal, a website where the applicants apply for approval of licenses, FSC and Registration numbers. In addition, the portal provides an interface and ease of access for applicants to track the submitted application, query responses and the ability to download permissions issued by the CDSCO.
Any applications submitted are reviewed and approved/rejected by the CDSCO. The process involves the submission of necessary documents on the online portal. After uploading the documents, applicants must submit a hard copy of the papers to the CDSCO (Medical Device Division) for further approval on the medical device portal.
How to Apply
- Step 1: An applicant (manufacturer/importer) requires a user login id with the credential to apply on the SUGAM portal (https://cdscomdonline.gov.in/NewMedDev/Homepage).
- Step 2: The registration requires uploading all the self-attested copies of the undertaking (the documents which include the name and address of the Company and issued by the Government Authority).
- Step 3: The applicant also has to upload Aadhar ID proof (Authorised person/agent), the manufacturing license or Wholesale Drug License and the certificate of incorporation.
- Step 4: After online registration, the applicant must submit hard copies of all the uploaded documents and the Cover Letter to the CDSCO for verification. The CDSCO will approve the Sugam Registration after evaluating the proposed application and the documents.
Form Names: MD12, MD13
Test license to Manufacture In-Vitro Diagnostics (Form MD 12, 13)
Looking to obtain a Test License to manufacture In Vitro Diagnostics in India? Look no further than CliniExperts, the expert regulatory consultants. Our team of experienced professionals can guide you through the entire process, from submitting your application in Form MD 12 or 13 to obtaining the necessary regulatory approvals. We understand that navigating the regulatory landscape can be complex, but our expertise ensures that your application is handled with efficiency and accuracy. Trust CliniExperts to help you bring your innovative IVD device to the Indian market.
Regulatory Body Requirement
CliniExperts can assist in obtaining a test license for manufacturing In Vitro Diagnostics (IVDs) in India. IVDs under Class A, B, C, or D can be manufactured in small quantities for various purposes such as clinical investigation, testing, evaluation, examination, demonstration or training. However, manufacturers must follow a specific set of procedures laid down by the CDSCO.
As the highest regulatory body in the country, the Central Licensing Authority maintains quality and standards for manufacturing any medical device. To manufacture a medical device, including IVDs, a manufacturer must apply to the Central Licensing Authority by submitting Form MD-12, stating the purpose for manufacturing the device. CliniExperts can guide manufacturers through this process and help obtain a test license in Form MD-13 through the online portal of the Ministry of Health and Family Welfare, which typically takes up to 30 days. Trust CliniExperts to help navigate the regulatory process and ensure compliance for manufacturing IVDs in India
How to Apply
The application is done with reference to MDR 2017. Registration on the Sugam portal is mandatory for applying for the test license
- To apply for a test license to manufacture In Vitro Diagnostics (IVDs) in India, the manufacturer must submit an application in Form MD-12 to the Central Licensing Authority. The form should state the purpose of manufacturing the IVD, which can be for testing, evaluation, demonstration, or training.
- Once the application is approved, the Central Licensing Authority will issue a test license in Form MD-13, which can be obtained through the online portal of the Ministry of Health and Family Welfare. The entire process of obtaining a test license can take up to 30 days.
- It is important to note that the manufacturer must follow the procedures laid down by the CDSCO for manufacturing IVDs and maintain the quality and standards as per the regulatory requirements.
Contact us
Please feel free to talk to us if you have any questions. We endeavour to answer within 24 hours.


