CliniExperts: Global Compliance, Noble Expertise
AUTHORIZED AGENT
When navigating national/international markets, having a reliable partner is essential. Our Authorized Agent services offer you a strategic advantage, streamlining regulatory processes and ensuring compliance across borders.
CliniExperts - Unlocking Global Opportunities
Your Pathway to Success with Our Authorized Agent Services
Ready to expand your global footprint with confidence? Get in touch with CliniExperts team today to discuss how our Authorized Agent services can elevate your international market endeavors.

Step confidently into the Indian life sciences market while ensuring full compliance. CliniExperts serves as your reliable "Authorized Agent for Medical Devices" in India, guaranteeing all essential import compliance for your products and simplifying the process of appointing an Indian distributor.
Manufacturer

Authorized Representative (CliniExperts)

Distributers

India Markets
Who is an Authorized Agent ?
A foreign entity lacking a physical presence in India, but interested in entering the Indian market, has the option to designate an authorized representative to oversee the registration and promotion of their products within India.
An "Authorized Representative" refers to an individual or organization based in India, formally authorized by the foreign entity. This designated representative assumes responsibility for overseeing the foreign entity's import and business operations in India, which includes ensuring adherence to all provisions.
The Authorized Representative is required to hold a valid wholesale license that pertains to the sale and distribution of products or services within India. Additionally, they should possess the capability to initiate an application to the specific regulatory body.

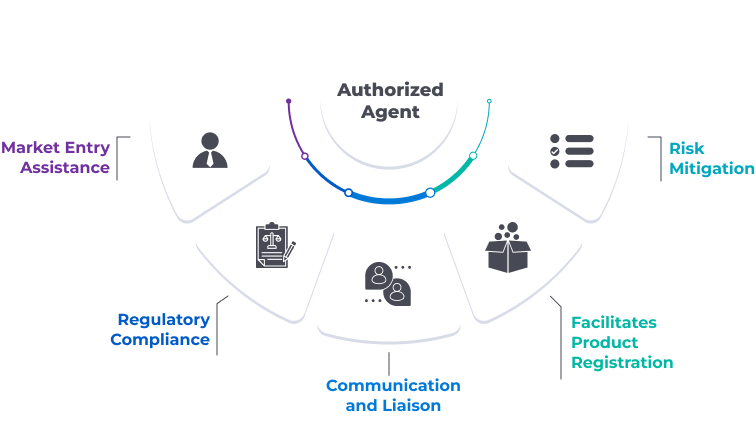
Why is an Authorized Agent required?
An Authorized Agent plays a crucial role in simplifying market entry, ensuring compliance, fostering local relationships, and enabling foreign companies to operate effectively in new markets.
Fig: Some key reasons why an Authorized Agent is necessary
HAVE A QUERY?
REACH US!INDIA OFFICE
CliniExperts Services Pvt. Ltd.
CliniExperts May assist you for:
- Authorized Agent Support
- Product Compliance
- License and Approvals
- Post Approval Changes
Product Categories Associated

Drug
Biologicals

Medical Device

IVD

Food

Cosmetics
 |
Medical Devices
|
 |
In-Vitro Diagnostics
|
 |
Drugs
|
 |
Food
|
 |
Biologicals
|
 |
Cosmetics
|
Our Happy Clients
Your Partner with 22+ Global Clients
Our Network Spans the Globe. Proudly serving as authorized agents for over 22 esteemed clients worldwide

Efficient team: Empowered by Our Expert Team of 40+
Our powerhouse team of 40+ seasoned professionals, dedicated to simplifying your market entry into India.
Trusted by Many: Handpicked Examples from Our Client Family


































CliniExperts – Your Personalized Authorized Agent
Foreign enterprises lacking a domestic footprint and aiming to explore the Indian market must designate an Authorized Agent.
We, CliniExperts possess a valid wholesale license in Form 20B or 21B, along with renewal license in Form 21C, making us an ideal choice as your Authorized Agent. We are well-equipped to serve as your in-country representative, managing the entirety of the registration procedures and liaising effectively with regulatory authorities.
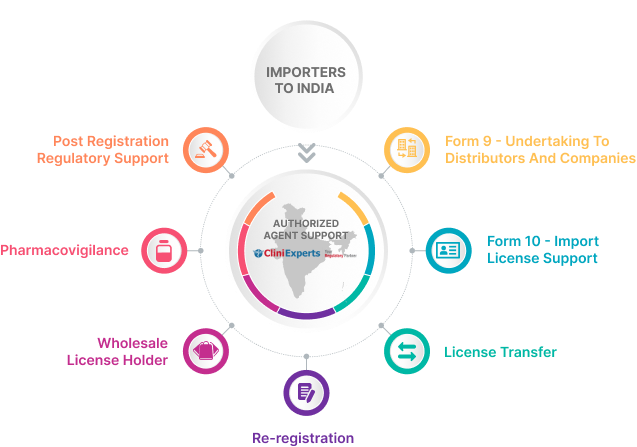
CliniExperts Service Bouquet

Import License Support
We help our clients obtain the Import License for Import swiftly by filing the documents to the regulatory authorities.

Custom Clearance
As the appointed authorized agent, we will assist you in placing orders, dispatching, and handling customs clearance and product delivery processes.

Post Registration Regulatory Support
With extensive regulatory expertise, we offer comprehensive post-registration support, including re-registration, license renewal, PSUR, ADR reports, label compliance, and more.

Pharmacovigilance
In collaboration with Pharmacovigilance companies, we help our clients monitor the effects of medical drugs and devices after approval and post marketing.

Re-Registration
We help our clients file for re-registration of their products well before the prescribed time so that the registration certificate may stay valid and doesn’t expire.

Warehouse and CFA Support
We streamline supply chains, cut costs, and boost customer satisfaction by efficiently managing the flow of goods from manufacturers to distributors.
Form Names: Form 19
Importer | Regulatory Body: State Licensing Authority (SLA)
Wholesale License (FORM 20B, 21B)
CliniExperts' professionals help you plan and streamline regulatory approval processes. Our hand in glove approach ensures that at no point you find yourself battling with any process by yourself.
Form Names: Form 14
Importer | Regulatory Body: CDSCO
Import License (FORM 15)
Get Your Import License today for Medical Devices and In-Vitro Diagnostic (IVD) Kits with CliniExperts. Meet all your Regulatory Compliance needs and get hassle-free assistance in providing Import License in Form MD 15.
Form Names: Form 12/ Form MD-16
Importer | Regulatory Body: CDSCO
Test License (Form 11/ Form MD-17)
CliniExperts' professionals know the latest laws and regulations related to the development, testing, manufacturing, approval, and commercialization of products and will provide complete guidance in securing a test license for importers or manufacturers looking to apply for Form 11 or Form MD-17.
Form Names: N/A
Importer | Regulatory Body: FSSAI
Food Product Compliance
We, the CliniExperts team are there for you for any assistance with completing your FSSAI food compliance for Food Supplements Nutraceutical and its registration aspects.
Form Names: Form 8
Importer | Regulatory Body: CDSCO
Biological Import License(Form 10)
CliniExperts helps you to get the permission to import biologicals in India. We cater end-to-end services from filling an application biological import license in Form 8 and getting approval in Form 10.
Testimonials
The Brand That Promises To Turn, Your Business Around!
Atomy India Pvt ltd
We have been associated with CliniExperts for about a year now for our food products related services for FSSAI compliances and licenses from CliniExperts and are completely satisfied with their performance. Quick turnaround time in responding to queries, meticulous planning in completing the task, alongside the extensive knowledge of the regulations of the entire team is highly appreciable and marks the professionalism and quality driven ethics of the organization. We highly recommend their services as we believe it is best in the industry so far.

Rajeev Rattava
Finance Manager
Morepen Laboratories Ltd.
The team at CliniExperts is proficient in the subject matter and provide prompt services . We are satisfied with our engagement and would recommend their services.

Anubhav Suri
Business Head
Proficon Medisol Pvt. Ltd.
We have been professionally associated with Cliniexperts for more than 5 years now. They have smoothly sailed us all these years in different projects & this has been possible because of the knowledge & dedication cliniexpert team has in their portfolio. All the best Cliniexpert. Keep going.

Ajitesh Trehan
Director
Unity Enterprises
We appointed Cliniexperts for our Cosmetic registration project for one of our critical products for which we received the registration certificate much before the timelines committed by the company. The entire project was handled efficiently with sheer professionalism and in our comfort zone . We are very happy with the overall working experience with CliniExperts and will highly recommend their services to everyone.

Faiyaz
Founder, Unity Enterprises
Frequently Asked Questions
Why is authorized agent support necessary?
To introduce a foreign company's product to the Indian market, the key requirement is the registration of products through an Indian entity. If foreign companies lack an Indian establishment or local partners, they must engage an authorized agent to manage the import of their products in India on their behalf.

What advantages will I gain by selecting CliniExperts as my authorized agent over my current Indian distributor?
To commence operations in India, you only require one Indian establishment. If you already have a distributor and decide to switch distributors later, you would need to repeat the registration process. With CliniExperts, registration is a one-time effort, allowing you the flexibility to add or change distributors as required without repeating the registration process.

In addition to serving as an authorized agent, what other services can you expect from CliniExperts?
CliniExperts provides a range of supplementary services, including:
- Import assistance and aid with customs clearance.
- Support for warehousing and distribution (CFA) of your products.
- Identification of suitable distributors to introduce your products in the Indian market.
- Assistance with post-market surveillance and pre-certification support.

What type of sales model does CliniExperts provide?
CliniExperts is a burgeoning business consultancy that addresses a comprehensive spectrum of your business requirements, encompassing license registration initiation, import assistance, and providing uninterrupted logistics support throughout the entire process.

What legal requirements must be met to access these services?
All legal requirements, such as pre-certifications, post-marketing surveillance inquiries, product recall requests, or any other necessary requests, are managed by the designated authorized agent.

