CDSCO Orders Regulation Of CT Scan Equipment, All Implantable Devices, Medical Equipment As Drugs

Regulations for the import or manufacture of medical devices
The Ministry of Health & Family Welfare, Government of India, has notified the list of equipment or devices which will be regulated as drugs. This regulation will be effective from April 01, 2021.
As per the order, the manufacturers or importers of medical devices and equipment like CT scan equipment, MRI equipment, defibrillators, PET equipment, dialysis machine, X-ray machine, bone marrow cell separator, and all implantable medical devices, will require an import/manufacturing licence from Central Licencing Authority or State Licensing Authority.
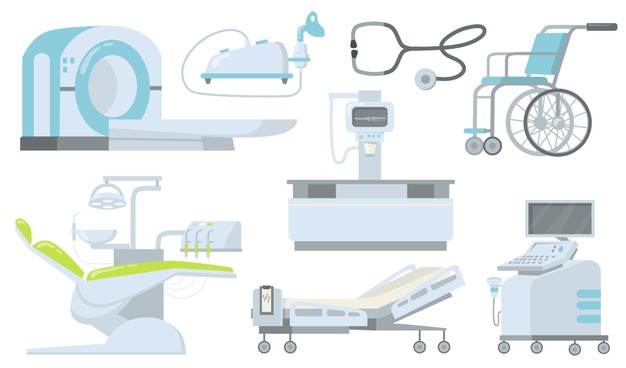
| Sr. No. | List of equipment and devices that will require import/manufacturing licence |
| 1 | All implantable medical devices |
| 2 | CT scan equipment |
| 3 | MRI equipment |
| 4 | Defibrillators |
| 5 | PET equipment |
| 6 | Dialysis Machine |
| 7 | X-ray Machine |
| 8 | Bone marrow cell separator |
Medical Devices Rules 2017
Rule 97 of Medical Devices Rules 2017 provides details for the rules being applicable. This applicability is in reference to various operations undertaken by the Drugs & Cosmetics Rules for the devices referred to in rule 2 of the Medical Devices Rules 2017.
Request for extension of the order implementation
Meanwhile, the CDSCO has received requests to extend the implementation of the notification for another 3 to 6 months. This request has been made due to the procedural work that needs to be done, like the resolution of queries, audit of various facilities by the notified bodies, and testing of product at the testing laboratories.
Grant of the import or manufacturing license under the provisions of Medical Devices Rules, 2017
It has been decided that the existing importer/manufacturer of these devices who has submitted the application for the grant of import/manufacturing licence to the Central Licencing Authority or State Licensing Authority, under the provisions of Medical Devices Rules, 2017, will be considered valid. Such importers/manufacturers can import/manufacture the device for up to 6 months from the issue of the order. The licence can also stay valid until the Central Licencing Authority or State Licensing Authority make decisions on the application.
Summary:
- The Ministry of Health & Family Welfare has notified medical devices to be regulated as drugs with effect from April 01, 2021.
- The existing importer/manufacturer who has applied for the grant of licence to the Central Licencing Authority or State Licensing Authority will be considered valid.
References
- Regulation of CT scan equipment, All Implantable Devices, MRI equipment, etc. as Drugs with effect from April 1st, 2021. Available at: https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=NzE0Ng== Accessed on May 14, 2021.
Recent Posts
Organic Food Labelling In India| Certification, and Import of Organic Food in India

This Article is All About Organic Food Labelling In India and Certification, and Import of Organic Food in India. Explained in Detail About What is Organic Food labelling? Summary Short Description Wi..
Cosmetic Label Compliance India : A Guide to Compliance

Introduction Looking for Cosmetic Label Compliance India? Are you a cosmetic manufacturer or importer navigating the complex world of Indian regulations? Ensuring your product labels comply with the l..
Clinical Investigation Approvals: An Overview of Forms MD-22 and MD-23

Summary Short Description Strict regulatory protocols govern clinical investigations for medical devices. Central to this process are forms MD-22 and MD-23. Form MD-22 is an application to Central Lic..
HAVE A QUERY?
REACH US!Office
New Delhi
Unit No. 324 & 325, City Centre Mall, Plot No. 5, Sector 12, Dwarka, India - 110075
+917672005050
Bengaluru
RMZ Galleria, 1st floor, Ambedkar Colony, Yelahanka, Bengaluru, Karnataka, India – 560064
Call us on
Sales: +91 7672005050
Reception: +91-11-45214546
Timings
9 am to 6 pm (Monday to Friday)


