Global Regulatory Services
We provide complete product life cycle management to our clients. Right from strategizing of products for specific markets, preparation and filing of appropriate documents to the Health Authorities of Regulated and Semi-regulated (Rest of the World- ROW) markets and post approval changes. We provide end to end support for all steps in your entry into the global markets.
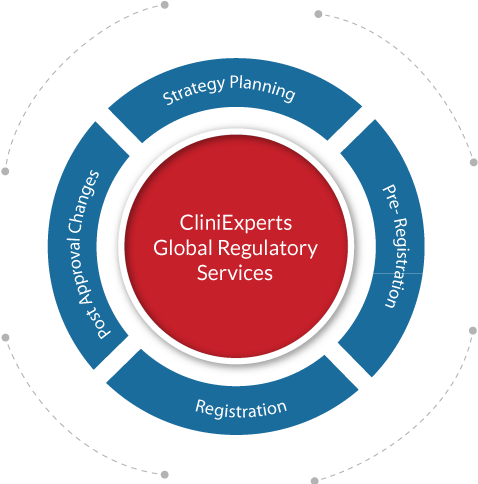
Strategy Planning Services
Aligning client’s market (domestic or international) with its business goals and product needs in-depth research and preparation of blueprint for filing regulatory dossiers. Our detailed and comprehensive Global Regulatory Affairs planning services ensure no snags at any level in your goal to product launch.
Assistance In
- Selection of geographical regions for the Registrations of Dossiers in different markets (Semi-Regulated and Regulated) based upon the availability of the data.
- Strategizing submission to developed countries, to reduce timelines for placement of products into export.
- Selection of geographical regions for the Registrations of Dossiers in different markets (Semi-Regulated and Regulated) based upon the availability of the data.
- Strategizing submission to developed countries, to reduce timelines for placement of products into export.
We provide advice management on the impact of current, newly finalized or proposed regulations, guidelines and standards and make recommendations accordingly.
Provide Regulatory Input To Product Lifecycle Planning
We provide operational direction in Product Lifecycle Planning, facilitating registration of products.
Monitor the impact of changing regulations on submission strategies
The labeling regulations of a semi-regulated market may update frequently depending upon the revisions in regulations. Based on updated regulations know how, we help our clients efficiently modify the strategy for submissions. It enables them to obtain hassle-free and speedy approvals from the country’s health authorities.
Periodic Safety Update Report (PSUR) solution for export registration
We provide Periodic Safety Update Report (PSUR) solutions for export registration in various countries and regions i.e. US, EU, Africa, Latin America, CIS, ASEAN, GCC and others. Our skilled team can write and review Periodic Safety Update Report as per country specific guidelines as applicable.
Pre-registration Services
We offer data generation, regulatory and quality compliance services with gap analysis and expert reports in line with the Global Licensing Regulatory of the specific country facilitating successful registration.
- GMP Compliance/Site Due Diligence for API Site, FP Sites
- Assessment of Regulatory documents and Existing dossier
- GMP Assessment and Due Diligence Analysis
- Gap analysis on the Existing documents/data/dossier and conversion to country specific/ACTD to CTD and vice versa
- Guidance & assistance on generation of adequate data for successful submission of the Dossier to Country MOH/Health authorities.
We assist our clients in development of new products and data generation in line with country specific registration guidelines/regulations.
Development & Preparation of R&D documents
We provide guidance in development and preparation of R&D documents like AMV Protocols and Reports, Stability Protocols and Reports, Product Development Report (PDR) in CTD (ASEAN, EU or ICH) format.
Comparative Dissolution Studies Designing and Data Report
We assist our clients in designing Comparative Dissolution Studies (with IVIVC) and in generating an acceptable data report for submission in the Health Authority. The summary of comparative studies of the product is required in the module 3.2.P.1 along with cross reference to the studies (with study numbers). It links the clinical formulations to the proposed commercial formulation and a successful correlation can assist in the selection of appropriate dissolution acceptance criteria.
Analytical Method development and Validation as per ICH Q2 (R1)
We have collaboration with certified labs for analytical method development, method validation and transfer services. These labs are capable of performing validation as per ICH guidelines, acceptable to MOH/FDA of ASEAN, African, CIS, Latin American and Rest of World (ROW). We handle the queries from MOH/FDA related to validation well. As your research partner, we provide detailed protocol, chromatograms and report for method development and validation at the conclusion of each Validation project which is acceptable at MOH/FDA during product registration.
We assist our clients in planning their BA/BE studies, obtaining NOC for BE from the intended country of export, and in conducting Bioequivalence (BE) and Bioavailability (BA) Studies with well-known CROs.
Reference Listed Drug (RLD)/Innovator Product/Comparator
We assist our clients in selection and identification of suitable Comparator/Reference Listed Drug product for the Generic applicants. Also, our team supervises and enables procurement/ import of Innovator Products required for the Comparative studies that helps in the generation of development data required for Dossier Registrations with MOH/FDA.
GMP Compliance/Site Due Diligence for API Site, FP Sites
At CliniExperts, our compliance consultants have a strong working knowledge of requirements and can help implement the necessary quality systems to meet the regulations. Additionally, we have substantial experience helping clients with remediation when their quality systems implementation and management fall short of meeting regulatory requirements.
Assessment of Regulatory documents and Existing dossier
We offer due diligence services for existing dossier and technical data for the dossier preparation as per the country specific requirements. We assist our clients in preparing the documents, using the regulatory compliant formats and closely monitor each step while working with them to fill the gaps for better Regulatory & Quality compliance. This minimizesthe queries raised during dossier assessment during GMP inspection by Country Health Authorities/MOH.
GMP Assessment and Due Diligence Analysis
GMP site inspection is one of the mandates for issuing of Registration Certificate by any Health Authority. We provide GMP consulting to proactively help our clients avoid issues with regulatory agencies enabling smooth registration process.
At CliniExperts, a detailed analysis is done of all the documents received from our clients. The loop holes observed as per country specific requirements are filled for strengthening the dossiers for better compliance. This enables them get speedy approvals.
Our team also ensures the conversion of dossiers to country specific requirements and from CTD to ACTD format or vice versa and verified for compliance.
We provide guidance & assistance by working closely with the technical team of clients towards the generation of adequate data for successful submission of the dossier to Country specific MOH/Health authorities.
Registration
We provide global regulatory solutions for International Dossier Registration in geographical regions worldwide providing support for DMF (CTD format) Preparation, Review and Submission, preparation of administrative documents, technical documents and dossier registration and handling query responses.

- DMF (CTD format) Preparation, Review and Submission
- Administrative Documents
- Technical Documentation (Writing and Review)
- Dossier Registration: Submission to various regulatory agencies worldwide
- Handling of Query Responses
DMF (CTD format) Preparation, Review and Submission
Our dedicated technical team of experts writes and reviews DMF/CTD as per ICH CTD/ASEAN CTD/Country specific guidelines. We also assist in the publishing and submission of USDMF and eDMF to USFDA. We assist our clients during the Product Registration till approval and also provide the support to address the MOH queries received for their other associated applicants, if needed.
Administrative Documents
We guide our clients on know-how about the kind of documents required for specific country registration. Along with we assist them to draft and finalize the country specific administrative documents/CTD Module-1. This includes (though, not limited to)- Letter of Authorization, Pack insert / PIL/Summary of Product labeling (SmPC/SPC), preparation of label and carton contents, justification for FDC, Global Patent Information, Literature for patented product and other administrative documents.
Technical Documentation (Writing and Review)
We assist our clients in preparation of USDMF/EDMF in CTD format, writing Quality Overall summary (QOS), and manufacturing and characterization part of DMF. We provide support in preparing documents for process validation, AMV and Stability Studies, Specifications, Method of Analysis (MOA) and Certificate of Analysis (COA) of Raw materials and API. We also assist them in query response to MOH/FDA.
Dossier Registration: Submission to various regulatory agencies worldwide
CliniExperts is a one stop for Global Regulatory Solutions which assists clients in seamless entry into Global regions by getting their products registered as per the expected timelines with minimal anticipated queries with Get –it-Right-at-first time approach. Support in full dossier writing as well as review of documents is done for companies interested in Pharma Export Business. We prepare, write, review the dossiers (as required) as per European/US CTD and ASEAN CTD and non-CTD/country-specific requirements and finally submit to respective Health Authorities.
Handling of Query Responses
We provide assistance in review and timely query response to the Regulatory Agencies/Health Authorities. A detailed discussion, planning and follow-up with the clients is done for the generation of additional data as required.
Post Approval Changes
We provide global regulatory consulting or assistance in Post-Approval Changes/Variation (minor, major, critical), assisting our clients to upgrade the existing products with additional data generation to the requirements of the countries. This includes planning, preparation and submission of the additional documents.
Providing regulatory intelligence and guidelines regarding updates pertaining to regulatory changes, timelines, and required data in the existing product dossiers of the clients.
Assistance in extension of labels and shelf life is also provided.
Related Services
Wholesale Drug License in India
Importer | Regulatory Body: SLA
CliniExperts offer strategic planning services for Wholesale Drug License to start with your pharmaceutical business and sell drugs at distributor level. Our hand in glove approach ensures that at no point you find yourself battling with any process by yourself.
Clarification Letter / No Objection Certificate for Medical Devices in India
Importer | Regulatory Body: CDSCO
Unclear about the regulatory status of Medical Devices in India. Let CliniExperts’ professionals assist you for getting a Clarification Letter / No Objection Certificate (NOC) for Medical Devices from Central Drugs Standard Control Organisation.
Test License for Medical Devices in India
Importer | Regulatory Body: CDSCO
Need a permission to import medical device in India to demonstrate its performance? CliniExperts’ professionals have expertise and assist you in securing a medical devices test license for importers in Form MD 17 by CDSCO.
Non-Notified Medical Devices Registration/ Approval in India
Importer | Regulatory Body: CDSCO
CliniExperts acts as an authorized representative to help you in getting the permission to import non-notified medical devices in India. Register your Non-notified Medical Devices in India with CliniExperts professional assistance
Authorized Agent For In-Vitro Diagnostic Kits
Importer | Regulatory Body: CDCSO
Importing an in-vitro diagnostic kit and selling it across the country can be overwhelming if you do not have any local establishments in India. With a well-established presence in India, CliniExperts can help you comply with CDSCO requirements and start selling your device in this emerging market. CliniExperts hold a drug wholesale license in Form 20-B and 21-B and can be your in-country representative and IVD importing a hassle-free process.
Permission to import or manufacture new medical device in India
Importer | Regulatory Body: CDSCO
Get experts assistance to avail Permission to import or manufacture medical device which does not have its predicate device in India -As per MDR 2017

Insight
Initiatives on Global Clinical Trials
India has been an attractive destination for clinical trials because of its large population and varied demographics. Despite having tremendous potential, only 1–2% of global clinical trials are being conducted in India. To utilize its full potential with respect to the trained GCP professionals, trained investigators as well as infrastructure, […]
Read MoreNews
A Blueprint for Helping Children with Rare Diseases
The U.S. Congress and the Food and Drug Administration have long focused on bringing new therapies to patients with rare diseases, including children.
Read MoreNews
Keeping You Informed: An Update on FDA’s Judicious Use Strategy for Antimicrobial Drugs in Food-Producing Animals
Today, “antibiotic resistance” is a widely recognized concern. With the rise of bacteria that are resistant to many, and in some cases, all standard treatments, scientists and medical professionals are not alone in focusing on this problem.
Read MoreNews
FDA Refines Its Thinking on Nanotechnology
FDA finalized three guidance documents that address various aspects of the use of nanotechnology in products regulated by the agency
Read MoreInsight
CDSCO has drafted guidelines for determining Quantum of financial compensation to be paid in case of Clinical Trial related Injury or Death
The Good Clinical Practice (GCP) Guidelines for Clinical Trials of India under para 2.4.7 provides that the research subject who suffers physical injury as a result of their participation in clinical trials are entitled to financial or other assistance
Read MoreNews
Pfizer, Bristol get EU nod for blood clot preventer
European health regulators on Tuesday approved an eagerly anticipated blood thinner developed by Bristol-Myers Squibb Co and Pfizer Inc for preventing strokes and blood clots in patients with an irregular heartbeat known as atrial fibrillation, the companies said.
Read MoreNews
Top Five Trends Changing the Game in Global Healthcare IT
stethoscope in two years, preferring to examine his patients with a handheld ultrasound unit to view a patient’s heart in real-time. In 2012 alone, physicians in the U.S. are expected to increase their use of smartphones and tablets by 81 per cent.
Read MoreStrategy
Global Licensing & Registration
Drug product registration and licensing in Europe, Africa, Asia, the Middle East and South America
Read MoreStrategy
Global Drug & Medical Device Regulatory Consulting
Global Licensing and Registration, Product Registration Process – a strategy that ensures optimum results, Dossier Preparation for Export and Registration, Writing & Review of- Summaries and Drug Master File
Read More