India Regulatory Services
CliniExperts - Your reliable partner for Comprehensive Compliance Solutions
REACH US
CliniExperts is the catalyst you need to speed up your registration process without worrying about obstacles. Our experience, professional relations understanding and wealth of practical familiarity in the field serve to ease your way through the process. With CliniExperts, you will receive expert advice, 24 hour support, practical guidance and complete technical assistance right from the beginning till the very end of your penetration into the Indian market.
Our Expertise

Product Categories
We offer regulatory solutions to Medical Device, Drug, Biologicals, Food, In-vitro Diagnostic and Cosmetics

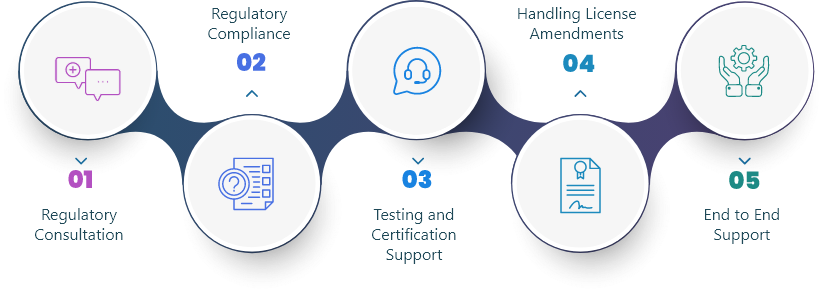
We Aid Our Clients In All Regulatory Matters
Regulatory bodies are organizations that have the responsibility to scrutinize, steer and manage different sectors in order to ensure the safety of consumers. Such bodies are formulated by the Government in sectors like the drug industry, cosmetic industry and food industry to regulate and control the quality of products that are made accessible to consumers through the market. Brief descriptions of some apex regulatory bodies that govern the Indian markets are:

CDSCO / Zonal CDSCO / State FDA
The Central Drugs Standard Control Organization (CDSCO) is a regulatory body that lays down stringent standards that any drug, medical devices, diagnostic kits, cosmetics, biologicals and vaccines being introduced into the country must meet before being sold in the market. CDSCO has 6 Zonal Offices which are headed by Dy. Drugs Controller (India).
The State Food and Drug Administration has branches in every state that serve to maintain safety and quality standards of food ingredients, food products and imported drugs. This is the highest governing authority for all food and drug clearances needing entry into the country and every state.
One of the key responsibilities of the CDSCO is to grant permits for specific crucial categories of drugs, including blood and blood products, intravenous fluids, vaccines, and sera. It ensures that these specialized drugs meet the necessary standards and are safe for use.
Its regulatory oversight helps to build trust and confidence in the healthcare system.
FSSAI
The Food Safety and Standards Authority of India (FSSAI) is a branch of the Ministry of Health and Family Welfare dedicated to standardizing food products made available in the market through a series of standards that ensure food safety.
It was created under the Food Safety and Standards Act, 2006 to ensure the safety and quality of food products in India.
The primary responsibility of FSSAI is to regulate and supervise the manufacturing, processing, distribution, sale, and import of food items to ensure they meet the defined standards for safety and quality. FSSAI sets food safety standards, and regulates the licensing and registration of food businesses in India.
ICMR
The Indian Council of Medical Research (ICMR) is the apex body in India for the formulation, coordination, and promotion of biomedical research. ICMR operates under the Department of Health Research, Ministry of Health and Family Welfare, Government of India.
It is the leading body regulating all medical research in the country. It essays a vital role in the prevention and control of communicable diseases, research regarding toxicology, immunology, reproduction, national medical statistics, food standards and other primary health topics of national interest.
The primary mandate of ICMR is to promote and coordinate medical research activities across the country. It plays a pivotal role in advancing scientific knowledge and addressing health challenges by conducting research, establishing research institutions, and promoting collaboration among researchers, institutions, and industries.
DSIR
The Department of Scientific and Industrial Research (DSIR) is an apex body in India that regulates and standardizes topics concerning scientific and industrial sectors of national importance. It also holds the power to grant recognition to public funded universities and is the controlling power over all the IITs and IISCs.
DSIR’s initiatives aim to create an environment conducive to research and innovation, strengthen India’s scientific capabilities, and foster the growth of industrial sectors through technological advancements. By promoting R&D and facilitating technology transfer, DSIR contributes to the overall development of science, technology, and industry in the country.
DGFT
The Directorate General of Foreign Trade (DGFT) is an agency of the Government of India that operates under the Ministry of Commerce and Industry.
The primary role of DGFT is to promote and regulate foreign trade in the country. It is responsible for issuing and regulating export-import licenses. It grants various licenses and authorizations, such as Importer-Exporter Code (IEC), Advance Authorizations, Export Promotion Capital Goods (EPCG) scheme, and Duty-Free Import Authorization (DFIA), among others.
It plays a pivotal role in granting licenses and authorizing trade transactions between India and other countries. It also administers various export promotion schemes to support and incentivize Indian exporters. It conducts investigations, audits, and inspections to prevent trade malpractices, monitor export-import transactions, and enforce compliance with trade laws.
NPPA
The National Pharmaceutical Pricing Authority (NPPA) is an organization in India that operates under the Ministry of Chemicals and Fertilizers.
The primary objective of NPPA is to regulate and control the prices of pharmaceutical drugs in the country. It has been set up by the government to control and regulate standardized prices for the drugs available in the Indian market.
It ensures that drugs that are needed by masses, and all essential drugs are affordable and easily available throughout the country. It works towards ensuring a balance between the interests of the pharmaceutical industry and the affordability of medicines for patients.
CIBRC
Central Insecticide Board and Registration Committee(CIBRC) is a division of Directorate of Plant Protection, Quarantine and Storage. CIBRC was set up by the Department of Agriculture and Cooperation in order to standardize the quality of insecticides available in the country in order to prevent danger. It ensures that all tenements of the Insecticide Acts and Rules are followed meticulously.
In the Act and the Rules framed there under, there is compulsory registration of the pesticides at the Central level and license for their manufacture, formulation and sale are dealt with at the State level. With the enforcement of the Insecticides Act in the country pesticides of very high quality are made available to the farmers and general public.
CBN
The Central Bureau of Narcotics (CBN) is an Indian government agency that operates under the Ministry of Home Affairs. It is responsible for the enforcement of the Narcotic Drugs and Psychotropic Substances (NDPS) Act in India.
The CBN issues licenses for the cultivation, production, and manufacturing of narcotic drugs and psychotropic substances for medical and scientific purposes. It conducts investigations, arrests, and seizures related to drug trafficking, production, and distribution.
It plays a vital role in curbing the illicit drug trade, preventing drug abuse, and protecting public health and safety in India.
About CliniExperts
your go-to Regulatory Solutions provider in India!
Established in 2009 by Dr. Ashwini Kumar, CliniExperts is a leading provider of Regulatory and Clinical Research Services. With ISO 9001 and ISO 27001 certifications, we prioritize strict adherence to all the rules and regulations and ensure top-notch standards in our services.
Our team of passionate and skilled professionals collaborates with global companies known for their high-quality products, services, and customer care. We specialize in assisting companies across various sectors, including Medical Devices, In-Vitro Diagnostics, Pesticides, Insecticides, Drugs and more.
At CliniExperts, we live by our belief in unwavering quality and pursuit of excellence. Our zero-tolerance policy for defects creates a flawless regulatory ecosystem, enhancing ease of doing business for our clients.
Choose CliniExperts as your trusted partner for regulatory compliance and streamlined approvals.
Why Choose CliniExperts?
End-to-End Regulatory Solutions for Domestic and International Markets
Certifications ISO 9001:2015 and 27001:2013




Our Happy Clients
We collaborate with companies operating in various sectors such as pharmaceuticals, biologics, medical devices, in-vitro diagnostics, food, nutraceuticals, cosmetics, and other related industries.









































































Testimonials

I want to extend my sincerest thanks for your diligent work and effort in submitting the claim approval application to the Regulatory Body. Your attention to detail and prompt communication have ensured that we remain on track and well informed throughout this submission process. We appreciate your continued support and professionalism. Thank you once again for your exceptional services.
Dr Nikhil Teli
Manager - Innovation, GCViDeal (A GCV Life Pvt. Ltd. Company)
We’re happy with the Regulatory services rendered and support by CliniExperts for our company as they execute the projects diligently keeping all the regulatory checkpoints in consideration to ensure all activities are carried out ethically by meeting the required MDR 2017 compliances and transparency, which is desired in all the projects. We received the approvals for our IVDs import Licenses in expedited timelines and the entire project was handled professionally and delivered under specified timelines by CliniExperts.
Nagaraj G P
Randox Labortaroies
We appointed Cliniexperts for our Cosmetic registration project for one of our critical products for which we received the registration certificate much before the timelines committed by the company. The entire project was handled efficiently with sheer professionalism and in our comfort zone . We are very happy with the overall working experience with CliniExperts and will highly recommend their services to everyone.
Mr. Faiyaz
Unity Enterprises
We have been associated with CliniExperts for about a year now for our food products related services for FSSAI compliances and licenses from CliniExperts and are completely satisfied with their performance. Quick turnaround time in responding to queries, meticulous planning in completing the task, alongside the extensive knowledge of the regulations of the entire team is highly appreciable and marks the professionalism and quality driven ethics of the organization. We highly recommend their services as we believe it is best in the industry so far.
Rajeev Kumar Rattawa
Atomy India Pvt ltd
Cliniexperts is one of the best consultants I have come across. It was extremely easy for me to get information from the team I found that the people working on this organization are full of knowledge and professional. Also the speed at which the information is provided was a real take for me to work with them. I will continue to work with them and will not hesitate to recommend them to any one who would need consultancy on regulatory front. It is always pleasure to work with such a professional team.
Naavin Changchroenkul
Suretex Limited
We have been associated with CliniExperts since 2018 . Quick turnaround time in responding to queries, meticulous planning in completing the task, alongside the extensive knowledge of the regulations of the entire team is highly appreciable and marks the professionalism and quality driven ethics of the organization. The entire team of CliniExperts follow a proactive and collaborative approach keeping abreast with regulatory guidelines , clear communication, and transparent operations . We highly recommend their services as we believe it is best in the industry so far
Jayakar Chandran
Leader Biomedical and Surgicals India Pvt Ltd.
We have been professionally associated with CliniExperts for more than 5 years now. They have smoothly sailed us all these years in different projects & this has been possible because of the knowledge & dedication CliniExperts team has in their portfolio. All the best CliniExperts. Keep going.
Ajitesh Trehan
Director – Proficon Medisol Pvt. Ltd.
We have been associated with CliniExperts since 2020 for Cosmetics Product Registration in India. We are very happy with the way they have handled the registration process! Their expertise in regulatory affairs, professionalism and commitment to timelines are an asset to any company looking to work with them. CliniExperts have a team of very efficient, talented and customer-service oriented professionals. Indeed, we have had a great experience with CliniExperts and highly recommend them.
Radhakrishnan V.K
Research & Development Manager, Omniscent Fragrances Fz-llc, Dubai, Uae.
We have been associated with CliniExperts for the last 5 years. During this tenure, we have found them very professional in their approach, good understanding of the subject with strong ethical and compliance standards. They adhere to the mutually decided timelines and keep us updated on the project on a regular basis. We thank them for all their support and wish them all the best.
Sathya
Managing Director – South Asia, Galderma India Pvt. Ltd.
Thank you very much for the superb service solidified on our various regulatory projects. Your knowledge, expertise and skills are the key factors for the successful delivery. CliniExperts got us the approvals for the projects which are very critical. That was fabulous job. We appreciate the efforts taken in delivering the assignments without much delay. Status Update, Documentation and Support rendered while processing the applications were remarkable.
Boddu Ganesh
Dy. Manager-Regulatory Dept, Eisai India, Vizag, AP.
Thankyou very much for the wonderful service rendered on our various regulatory projects. We appreciate the efforts taken in delivering the assignments without much delay. Status Update, Documentation and Support rendered while processing the applications were phenomenal. Your knowledge, expertise and skills are the key factors for the successful delivery. One product registration was really critical. Our product was lying in the port and we were in need of a cosmetic registration urgently to clear the stock. In minimum span of time we got the Registration done and cleared the stock from the port without much demurrages. That was a commendable job.
Sunitha
Conybio Healthcare (India) Pvt. Ltd
We have had a great experience with CliniExperts. Their expertise in handling the Indian regulatory environment makes them the perfect partner. All our requirements were taken care of swiftly and on time.
Yash Shrivastav
Intercorp Biotech Limited
CliniExperts team is efficient, understanding, cooperative with Superchem and FBO’s to attend each requirement and query relating to product approval system as well as regulatory authorities. They are great help to us. Our best wishes for CliniExperts.
R.L Goyal
C.E.O (Superchem Nutri Formulations)
I just want to say Thanks! To the entire CliniExperts team for providing the outstanding support to help us to understand the various Food Regulation & to reply the various FSSAI queries letters for Product approval applications on time. You people are simply great, very alert; very approachable; I’m very fond of your way of working with FSSAI & your scientific justification & literature to resolve the various FSSAI queries professionally. In addition to being very friendly. CliniExperts are simply the Best Regulatory partner anyone can have for various regulatory supports. Once again Thank you to all CliniExperts team for having resolved my FSSAI tricky problems and for your wonderful support.
Pawan Shukla
Regulatory Affair Manager (Valentine Agro Pvt. Ltd.)Blogs
On October 1, 2023, the Medical Device Rule 2017 will go into effect, and manufacturers and importers of Class C&D devices will...
Regulation/Guidelines
October 25, 2023This Article is All About Organic Food Labelling In India and Certification, and Import of Organic Food in India. Explained in Detail...
Regulation/Guidelines
April 21, 2025Introduction Looking for Cosmetic Label Compliance India? Are you a cosmetic manufacturer or importer navigating the complex world of Indian regulations? Ensuring...
Regulation/Guidelines
March 26, 2025Summary Short Description Strict regulatory protocols govern clinical investigations for medical devices. Central to this process are forms MD-22 and MD-23. Form...
Regulation/Guidelines
January 15, 2025Navigating Software as a Medical Device regulation involves addressing varying global standards, including risk-based classifications.
Regulation/Guidelines
November 4, 2024The future of Software as a Medical Device will see breakthroughs in Artificial Intelligence-based diagnostics, personalised treatments, and remote monitoring.
Regulation/Guidelines
November 4, 2024Software as a Medical Device refers to software applications designed for medical purposes, such as diagnosis, treatment, or monitoring, without being part...
Regulation/Guidelines
November 4, 2024Software as a Medical Device faces many challenges related to quality management systems.
Regulation/Guidelines
October 22, 2024Clinical validation is an integral part of clinical evaluation of an SaMD, which is defined as a set of ongoing activities conducted...
Regulation/Guidelines
October 22, 2024Explore the various types of FSSAI licenses available in Delhi. Learn which license is right for your food business and how to...
Regulation/Guidelines
August 13, 2024Schedule M is a part of the Drugs and Cosmetics Act, 1945. It outlines the good manufacturing practices and requirements of premises,...
Regulation/Guidelines
June 28, 2024The Food Safety and Standards (Approval for Non-Specified Food and Food Ingredients) Regulations, 2017, initially established by the FSSAI, served as a...
Regulation/Guidelines
May 30, 2024Under the Food Safety and Standards, 2006, which consolidated numerous acts & orders that had previously addressed food-related issues in various Ministries...
Regulation/Guidelines
April 25, 2024One must obtain a drug license to launch a business selling drugs or cosmetics. No one can sell drugs or cosmetics without...
Regulation/Guidelines
February 14, 2024A wholesale drug license is required for manufacturing, distribution, sale, or storage of drugs. The Drugs and Cosmetics Act, 1940 outlines the...
Regulation/Guidelines
February 14, 2024The Central Pollution Control Board (CPCB) issued a directive alert for submission of timely annual reports by all Pollution Implementing and Beneficiary...
Regulation/Guidelines
December 5, 2023The Central Drugs Standard Control Organization (CDSCO) has announced that effective from the 1st of October 2023, Class C and Class D...
Regulation/Guidelines
September 4, 2023The national regulatory body for regulatory functions and processes in India goes by the name of Central Drug Standard Control Organisation (CDSCO)....
Regulation/Guidelines
July 19, 2023Registration of Foreign Food Manufacturing Facilities (ReFoM) intending to export Egg powder; Nutraceuticals; Meat & meat items, including fish, poultry & their...
Regulation/Guidelines
May 2, 2023Central Drugs Standard Control Organization (CDSCO) is the regulatory body of India regulating medical devices, drugs, and cosmetics in the Indian market....
Regulation/Guidelines
April 28, 2023Enquire Now
Please feel free to talk to us if you have any questions. We endevor to answer within 24 hours.



