Biological Registration Certificate in India - Form 40 & Form 41

Make the Biological Registration Process streamlined with CliniExperts. Let our experts help you with the Biological Registration Certificate. We follow the strategies for successful biological product registration with DCGI. The process involves filing Form 40 and obtaining the necessary license in Form 41. This enables you to import your products into India.
Biological Registration Form 40 & 41 – Overview
If a company from another country wants to register their manufacturing facility and sell biological products in India, they must get a certificate. Navigating the Drug Controller General of India's (DCGI) approval process for biosimilars requires a comprehensive understanding of its intricate details. The regulatory pathway for biosimilar approval in India involves a rigorous evaluation of clinical data, manufacturing processes, and comparative studies to ensure the safety and efficacy of these complex biological products.
In India, the Central Drugs Standard Control Organization (CDSCO) is in charge of issuing this certificate. To obtain the registration certificate, the applicant must register on the SUGAM portal. This application should be made in Form 40, uploading all the required documents, and by paying the government fees. Applicants need to fill out certain forms (called undertakings) listed in Schedule D-I and D-II. They also have to submit the required documents according to the Drugs and Cosmetics Act and Rules.

Who Can Apply?
Indian Applicant on behalf of Foreign Manufacturer
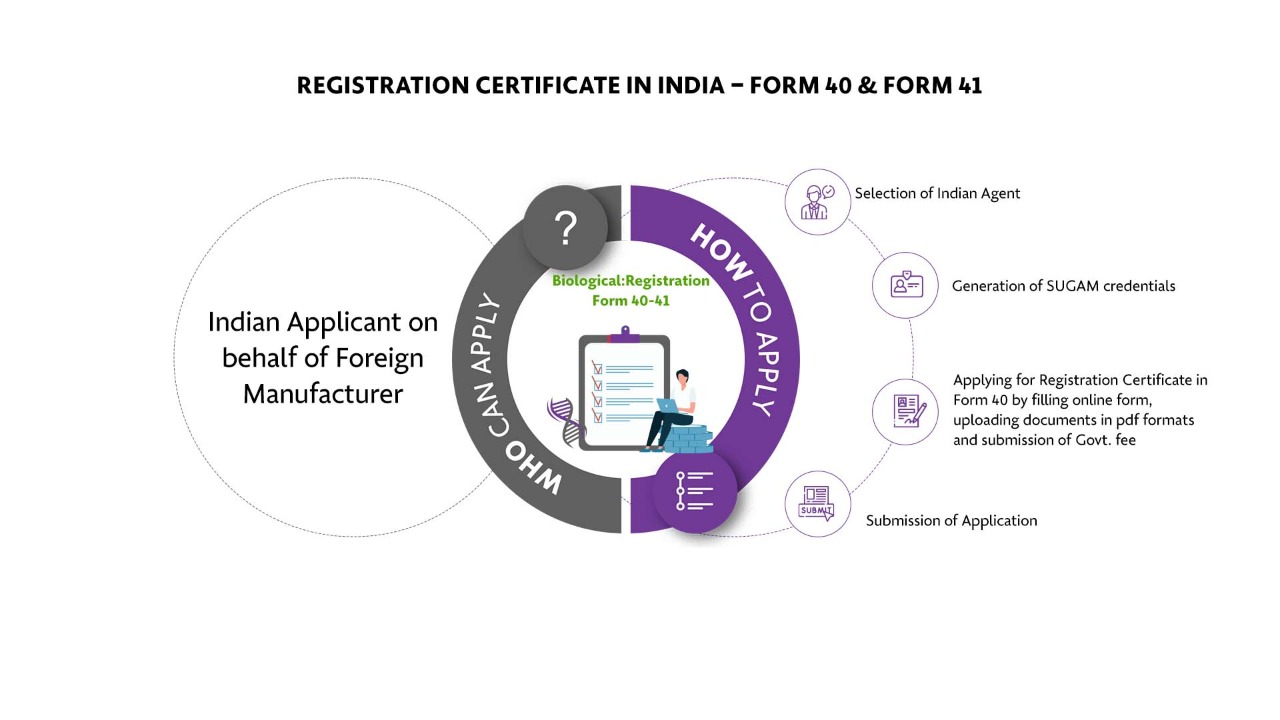
How To Apply?
The Applicant must follow the following process:
-

Selection of Indian Agent
-

Generation of SUGAM credentials
-

Applying for Registration Certificate in Form 40 by filling online form, uploading documents in pdf formats and submission of Govt. fee
-

Submission of Application

Validity
The registration certificate will be valid for three years.

Fee Involved
The cost for a Biological Registration Certificate is $10,000 for each manufacturing site and $5,000 for each drug. This fee is for registering the facility and importing biological products into India.Important Documents

Documentation requirements for CDSCO biological product approval are mandatory to facilitate the approval process. The following documents are required while taking the permission for registration of manufacturing premises and the product meant for import and market in India:
- Wholesale license and/or manufacturing license.
- Drug master file
- Site master file
- Free sale certificate/Certificate of Pharmaceutical Product
- Good Manufacturing Practice Certificate
- Power of attorney
Timeline to get
Form 41
from CDSCO
9
MONTHSEssential Tips
To successfully obtain a registration certificate for their manufacturing premises and to import and use biologicals in India, it is crucial to adhere to CDSCO guidelines for biological product registration, and also, clinical trial protocols for biologics in India should be followed. The applicant must ensure the following points as well:
- Stability data of at least six months is available at the time of application.
- Must have new drug approval from the regulatory authority India.
- The documents submitted while making an application must be notarized or apostilled.
- The indication/strength/dosage form/ route of administration of the approved product should not be changed.
- Documents submitted by the applicant or manufacturer must have correct details of name and address.
Expert Advise
The manufacturer must check if the manufacturing site they are applying for has not been granted a registration certificate before.
The manufacturer must see that all the regulatory documents are within the validity period.
The product samples sent for testing at the government laboratory must be well within the shelf life and in sufficient quantity.
Frequently Asked Questions
What is the difference between Form 40 and Form 41?
Form 40 is a specific application form for Import License while Form 41 is a specific approval form for Registration Certificate.

Should an individual get a registration certificate for biologicals that will not be sold or distributed in India but will be transited from India to other countries?
No, registration certificate is not needed in such cases of only transit.

When is the manufacturer required to obtain a fresh registration certificate?
The manufacturer must obtain a fresh registration certificate in the following conditions. These conditions are laid down by the Drugs and Cosmetics Act, 1940.
- The address of the Indian agent in the certificate or registered manufacturer in the certificate has changed.
- The name of the foreign manufacturer or Indian manufacturer mentioned in the registration certificate has changed.
- The Indian agent or the constitution of the manufacturer mentioned in the registration certificate has changed.

In a case where the firm's constitution and the manufacturing site's address operating under form 41 has changed, how long will the registration certificate remain valid?
The registration certificate will remain valid for three months from the date of the change. Necessary new registration certificate must be applied for with the new address.

Who is the Licensing Authority for Wholesale license?
State Licensing Authorities.

Will the applicant be required to pay any fees in case of post-approval changes?
The manufacturer will be required to pay additional fees if the post-approval changes will affect the following:
- Registration certificate.
- The manufacturing process of the product.
- Packaging or labelling of the product.
- Testing or documentation of the product.

When will the manufacturer with a registration certificate not require paying any fees?
The manufacturer should not pay any additional fees in minor cases like the following:
- change in the batch size
- change in the source of API or excipients
- tightening of test limits to existing tests
- inclusion of warnings in package inserts

What are the key challenges in Indian biological drug registration process?
Key challenges in the biological drug registration process include the complex regulatory landscape, stringent documentation requirements, ensuring compliance with Indian biotech product regulations, managing costs and time constraints, and accurate interpretation of guidelines.

What is the Biological Registration Certificate renewal process in India?
The Biological Registration Certificate renewal process in India involves timely application submission before the certificate expires. Companies must provide updated documents, demonstrate compliance with regulations, undergo inspections, pay renewal fees, and await approval from the Central Drugs Standard Control Organization (CDSCO).

