Marketing Authorization/ New vaccine Approval - Importer in India - CT-18 & CT-19, CT-20

Looking to import biologicals for sale in India? Let CliniExperts handle your licensing process efficiently. With a decade of experience, our regulatory specialists ensure prompt approvals, simplified knowledge, and cost-effective solutions. Trust us for a seamless journey towards obtaining your import marketing authorization license. Contact us for a free consultation.
Marketing Authorization/ New vaccine Approval - Importer – Overview
Who Can Apply?
Application for market authorization is available to importers/Indian agent of biological products already having wholesale License in Form 20B/21B.
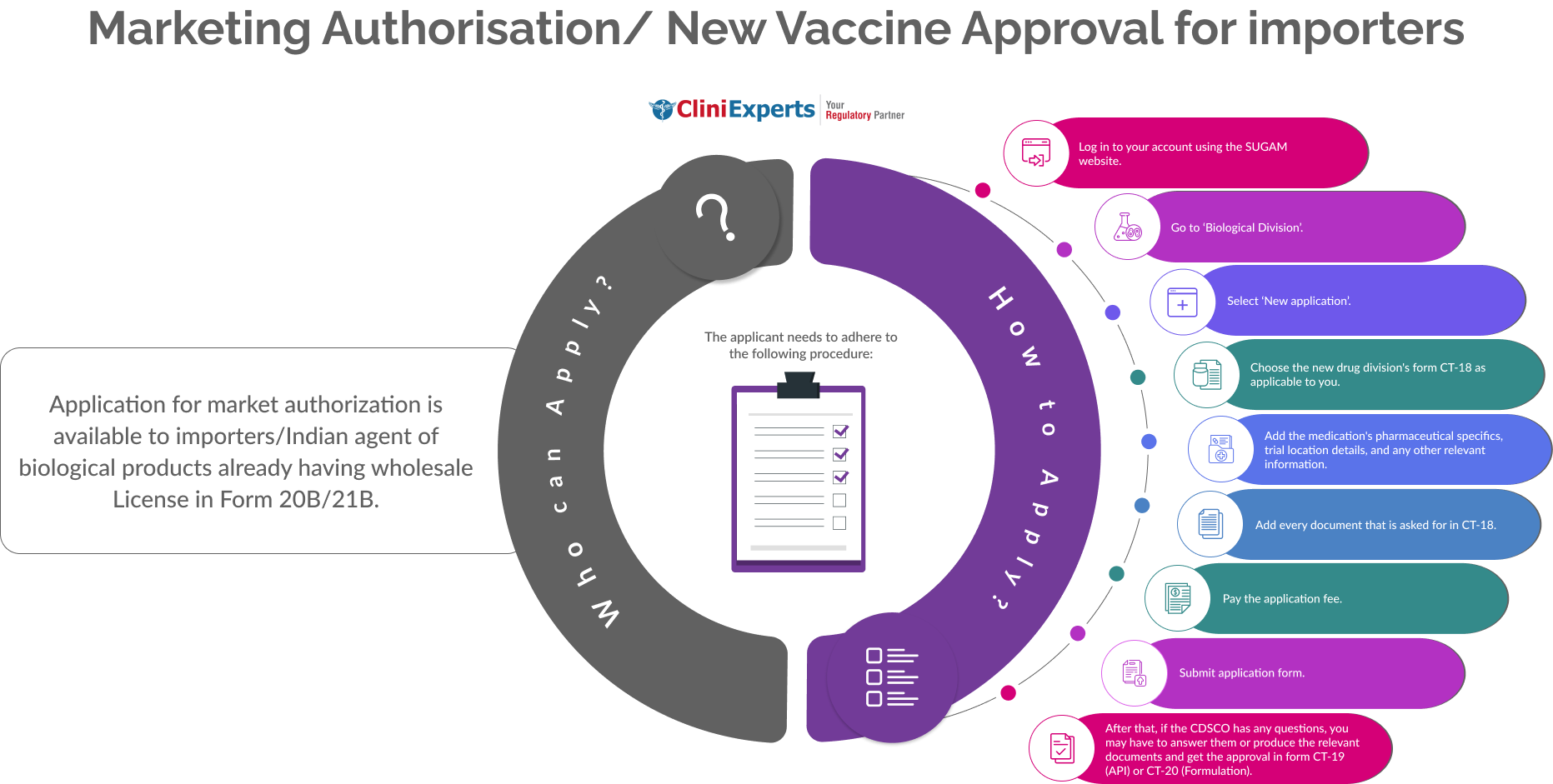
How To Apply?
The Applicant must follow the following process:
-

Log in to your account using the SUGAM website.
-

Go to ‘Biological Division’.
-

Select ‘New application’.
-

Choose the new drug division's form CT-18 as applicable to you.
-

Add the medication's pharmaceutical specifics, trial location details, and any other relevant information.
-

Add every document that is asked for in CT-18.
-

Pay the application fee.
-

Submit application form.
-

After that, if the CDSCO has any questions, you may have to answer them or produce the relevant documents and get the approval in form CT-19 (API) or CT-20 (Formulation).

Validity
There is a government fee of Rs. 5,00,000 for the application process.
Fee Involved
There’s no validity of the license mentioned. It remains valid for eternity unless cancelled or suspended for any reason.Important Documents

- Manufacturer and sponsor commitments and declarations.
- CMC data (containing drug master file, manufacturer's details, drug product details, site master file, COAs and batch stability data of three batches in which two should be of scale up size & one may be smaller).
- Study reports and summaries of approved preclinical studies (PK/PD and toxicology).
- Clinical studies reports and summaries for phase I, II, and III trials conducted.
Timeline to get
CT-19, CT-20
from Central Drugs Standard Control Organisation
90
DaysEssential Tips
- Provide clinical and pre-clinical data for phases I, II, and III.
- Send in all administrative documents (Manufacturing License, GMP), or explain if you are unable to.
- Provide CMC data, including three batches of COAs, and stability information for 3 batches.
Expert Advise
The documents like DMF, SMF & other administrative documents should be apostiled/notarized.
Every document provided needs to be authentic.
NDCT rule 2019 needs to be appropriately followed by all undertakings, including ICF, IB, labels, etc.
Frequently Asked Questions
Is it permissible for an importer to advertise a new medication for a condition that has not received approval?
No. Indian Government has banned the any kind of advertising in the country unless it is related to any government national health scheme. A new medicine's importer is never allowed to advertise it for a purpose that it hasn't been approved by the CLA. The package information and advertising materials for the new drug should not contain any indication of this kind.

What likely prerequisites need to be satisfied before the importer is allowed to sell a novel medication in India?

Which regulatory body oversees the import of veterinary medicines for use in India?
The veterinary cell of CDSCO is responsible for approvals of the import of veterinary medicines in India.

