Manufacturing License for Biologicals in India - 27/27A & 28/28A

With over a decade of regulatory compliance experience, CliniExperts provides complete regulatory solutions. Our team is well-versed in the latest industry practices. We have extensive connections to speed up the acquisition of licenses and approvals for your biologicals.
Manufacturing License for Biologicals – Overview

Who Can Apply?
The application is open to any new Indian entity that wishes to manufacture biological goods in India.
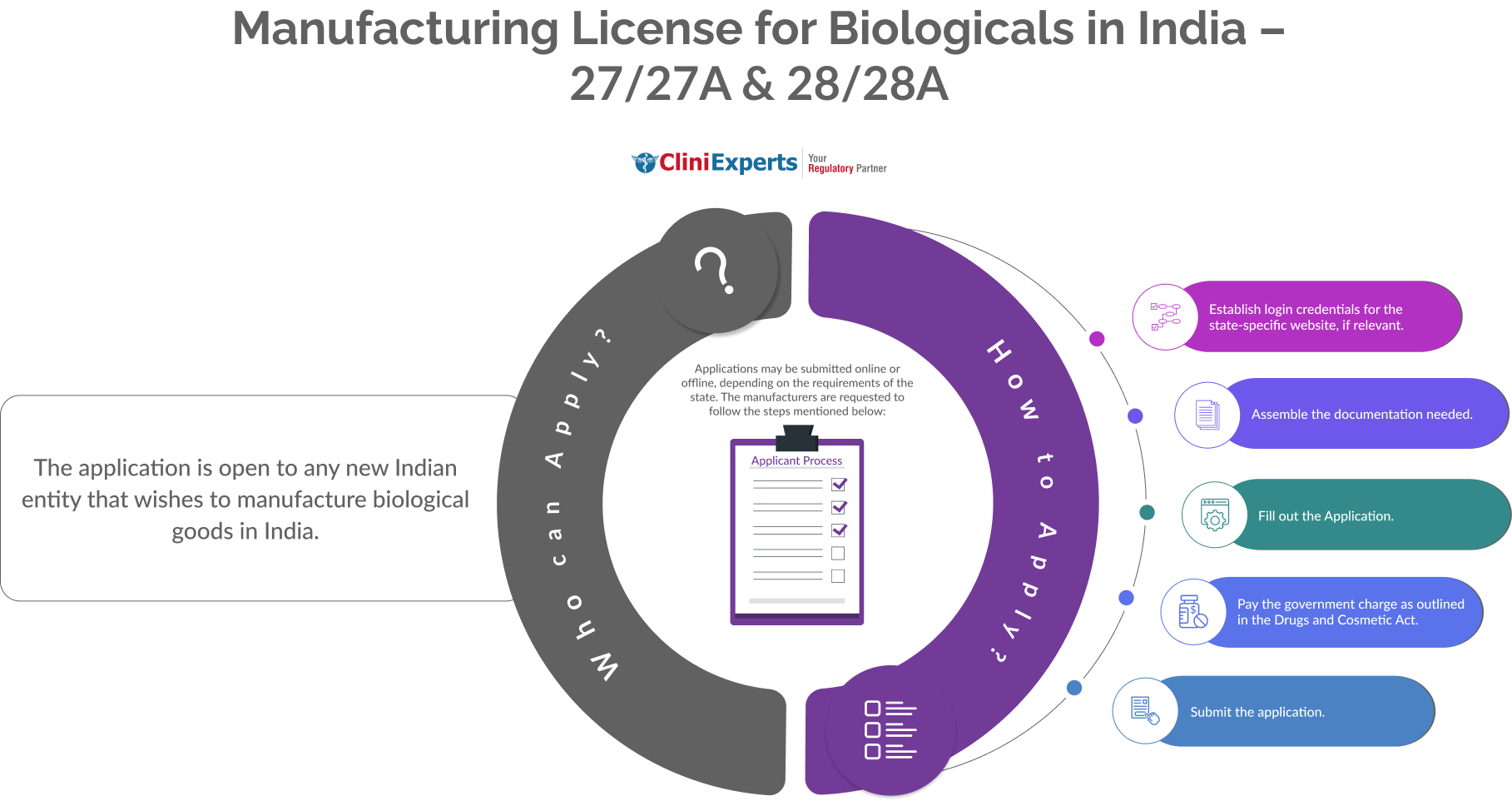
How To Apply?
The Applicant must follow the following process:
-

Establish login credentials for the state-specific website, if relevant.
-

Assemble the documentation needed.
-

Fill out the application.
-

Pay the government charge as outlined in the Drugs and Cosmetic Act.
-

Submit the application.

Validity
The license validity for manufacturing biological products is 5 years.

Fee Involved
There is a government fee of Rs. 7500 for the application process.
Important Documents

- List of SOPs
- List of machinery & Equipment
- Application Form
- Site master file
- Plant layout
- List of technical persons with qualification and experience
Timeline to get
28/28A
from Central Drugs Standard Control Organisation
60
DaysEssential Tips
- The documents submitted must follow the authority's provided checklist.
- The manufacturers should acquire an NOC from the pollution Control Board and fire department.
- The facility should comply with the Schedule M1 requirement.
- The obstacles or issues that are most likely to arise when filling out or applying for the manufacturing license are:
- Failure to acquire the NOC from the fire department and pollution control.
- The layout and site master file not provided in the specified order.
- Non-acceptance of the chemists.
Expert Advise
Facility should comply the Schedule M1 requirement.
NOC from the pollution Control Board & fire department is must.
Related Services
Test license to import In Vitro Diagnostics (Form MD 16, 17)
Importers | Regulatory Body: CDSCO
Do you need permission to import in-vitro diagnostic kits to India to demonstrate its performance? CliniExperts’ professionals have the expertise and assist you in securing in-vitro diagnostic kits test license for importer.
Permission to conduct Clinical Performance Evaluation (Form MD 24, 25)
Manufacturer / Importer | Regulatory Body: CDSCO
CliniExperts is backed by a well-informed and experienced team who guides through all the documentation and paperwork required for the regulatory processes.
Permission to Manufacture Class C & D In- Vitro Diagnostics (Form MD 7, 9)
Manufacturer | Regulatory Body: CDSCO
At CliniExperts, our team of experts is thoroughly updated with the latest requirements of the various authorities, enabling complete and meticulous documentation, timely application and quick approvals/licenses.
Permission to Manufacture Class A & B In Vitro Diagnostics (Form MD 3, 5)
Manufacturer | Regulatory Body: CDSCO
The application for manufacturing, sale or distribution of any medical device includes a tedious list of processes and forms CliniExperts team consists of experts working in the same domain with practical experience and knowledge of the governing rules.
Frequently Asked Questions
What is the primary requirement for the license application?

List down the documents required for the application process.
During the documentation process, the following documents should be present:
- List of SOPs
- List of machinery & Equipment
- Application Form
- Site master file
- Plant layout
- List of technical persons with qualification and experience

How much fee the government charge from the manufacturers for the application process?
There is a government fee of Rs. 7500 for the application process.

What is the government timeline to obtain License?

Can a manufacturer formulate a biological product after getting License?
No, the manufacturers must obtain product permissions and may also seek GMP certification.

