Marketing Authorization for Manufacturers of Biological Products in India - CT-21 & CT-22, CT-23

If you are looking to manufacture Biologicals in India, CliniExperts is your one-stop shop for handling the crucial licensing process successfully. Our dedicated team of seasoned regulatory specialists, who have over ten years of experience have mastered the subtleties of the typical obstacles and how to overcome them. We guide you through each stage to ensure a smooth road offering.
Marketing Authorization for Manufacturers of Biological Products – Overview
- Apply: CliniExperts will submit Form CT-21 to the Central Drugs Standard Control Organization (CDSCO) to start the application process for manufacturing biological products.
- Documentation: CliniExperts can support in completion and submission of all required documents as specified by CDSCO.
- License Approval: CDSCO will issue the marketing authorization license on Form CT-22 (API) and CT-23 (Formulation)

Who Can Apply?
CliniExperts Services can support Biological Product Manufacturers with existing valid license on Form 25 / Form 28 D in applying Marketing Authorization with CDSCO.
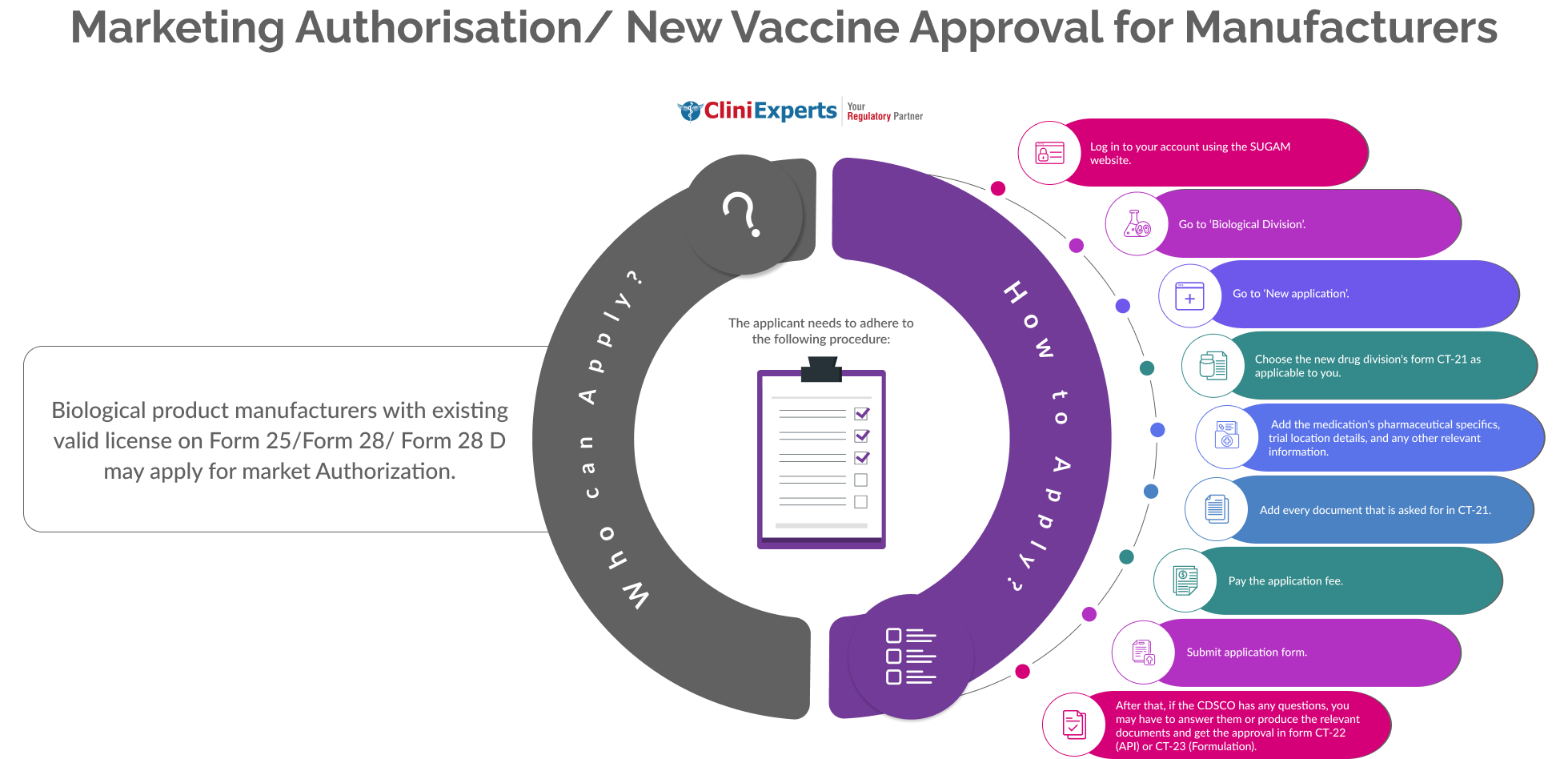
How To Apply?
The Applicant must follow the following process:
-

Log in to your account using the SUGAM website.
-

Go to ‘Biological Division’.
-

Go to ‘New application’.
-

Choose the new drug division's form CT-21 as applicable to you.
-

Add the medication's pharmaceutical specifics, trial location details, and any other relevant information.
-

Add every document that is asked for in CT-21.
-

Pay the application fee.
-

Submit application form. After that, if the CDSCO has any questions, you may have to answer them or produce the relevant documents and get the approval in form CT-22 (API) or CT-23 (Formulation).

Validity
The license mentioned has no validity. Unless it is cancelled or suspended for any reason, it is perpetually valid.
Fee Involved
Government charge for Market Authorisation would be INR 2 lacs - INR 5 lacs.Important Documents

- Declarations and commitments from manufacturers and sponsors.
- CMC data (including a master file for the drug, a manufacturer's file for the drug product, a site master file, a certificate of analysis (COA) and batch stability data for three batches).
- Summaries of study reports of preclinical research that have been approved (PK/PD and toxicity).
- Reports and summaries of clinical investigations related to phase I, II, and III trials.
Timeline to get
CT-22, CT-23
from CDSCO after submission of application
90
DaysEssential Tips
- Clinical and pre-clinical data for Phases I, II and III. (If not available, CliniExperts can assist you in perforiming clinical trial studies)
- All the necessary documentation in the correct and accurate format (Manufacturing License, Compliance to Good Manufacturing Practices), or provide justification if it is not available.
- Stability data and CMC data, including three batches of COAs.
Expert Advise
For a rapid and smooth license approval, Manufacturers must ensure (As Advised by CliniExperts):
NDCT rule 2019 must be adequately adhered to by all projects, such as labelling, IB, and ICF.
Each document that is submitted must be genuine.
All the documents should align with checklist provided by CliniExperts for SUGAM application process.
Why Choose CliniExperts?
By partnering with CliniExperts, manufacturers benefit from a streamlined, expert-led approach that minimizes the complexity of regulatory compliance, enhances the accuracy of your application, and accelerates your entry into the Indian market.

Expert Guidance
CliniExperts assist with the registration process with regulatory bodies and ensure compliance with all regulatory requirements, including the timely submission of Marketing Authorization application (Form CT 21) and associated necessary documents.

Thorough Document Review
CliniExperts conduct a meticulous review of all checklist documents, perform a GAP Analysis to identify and address any deficiencies, and communicate any issues promptly to ensure a smooth application process.
Final Review and Fee Management
CliniExperts oversee the final review of your Marketing Authorization application and coordinate the payment of government fees, ensuring that all financial aspects are handled efficiently.

Timely Follow up
CliniExperts will maintain timely follow-ups with the relevant Government bodies to keep everything on track.
Regulatory Compliance
CliniExperts ensure that all submissions are in strict adherence to the regulatory guidelines as per Drug and Cosmetics Act.
Frequently Asked Questions
Manual sign and stamp will be accepted or not?
Digital signature on the Form CT-18/CT-21 is mandatory.

Whether Clinical studies are required for product that are already marketed in India?

Who can apply for grant of permission to import and market a new drug?
Any person/ organization having a valid wholesale license for sale and distribution of drugs under The Drugs and Cosmetics Rules, 1945 can make application to CLA for grant of permission to import the new drug.

Is an importer or manufacturer allowed to market a new drug for unapproved indication?
No. Under any circumstances, the manufacturer or importer of new drug cannot market a new drug for an indication not approved by CLA. No such indication should be mentioned in the package insert/ promotional literature of the new drug.

Whether notarized GMP/CoPP/Mfg Lic required?
All the certificate such as GMP/CoPP/Mfg Lic required to be notarized.

I don't manufacture any medications right now. I want my facility to produce new drugs for the first time. How can I proceed?
In this case, the person or organization that constructed the manufacturing facility may apply to CDSCO for permission to manufacture novel medications for retail and distribution. However, in accordance with The Drugs and Cosmetics Rules, 1945, he must further apply to the appropriate State Licensing Authority for a manufacturing license.

Can a business legally market a novel medication for a condition that hasn't been approved by the FDA?
No. The producer is not allowed to commercialize a new medication for a medical condition for which it has not acquired CLA approval. The package information and advertising materials for the new drug should not contain any indication of this kind.

Why Government fee is lesser than 5 Lacs?
The fee rule has been updated as per GSR 1193. Click here to know more

