Test License to Manufacture Biologicals in India - Form 30 & Form 29

Do you seek a test license to manufacture drugs for purposes of examination, test or analysis? CliniExperts is your 360-degree solution to commercial success. We will handhold you through every step of submission of your Form 30 application to guarantee a seamless, delay-free journey to obtain the approval of Form 29 license, in compliance with the DCGI and SLA Guidelines.
Test License to Manufacture Biologicals – Overview

Who Can Apply?
Any manufacturers in India with a manufacturing license in Forms 25 and 28 or R&D units with DSIR can apply for a test license to manufacture test batches for the purpose of generating chemistry, manufacturing, and control data for further regulatory approval.
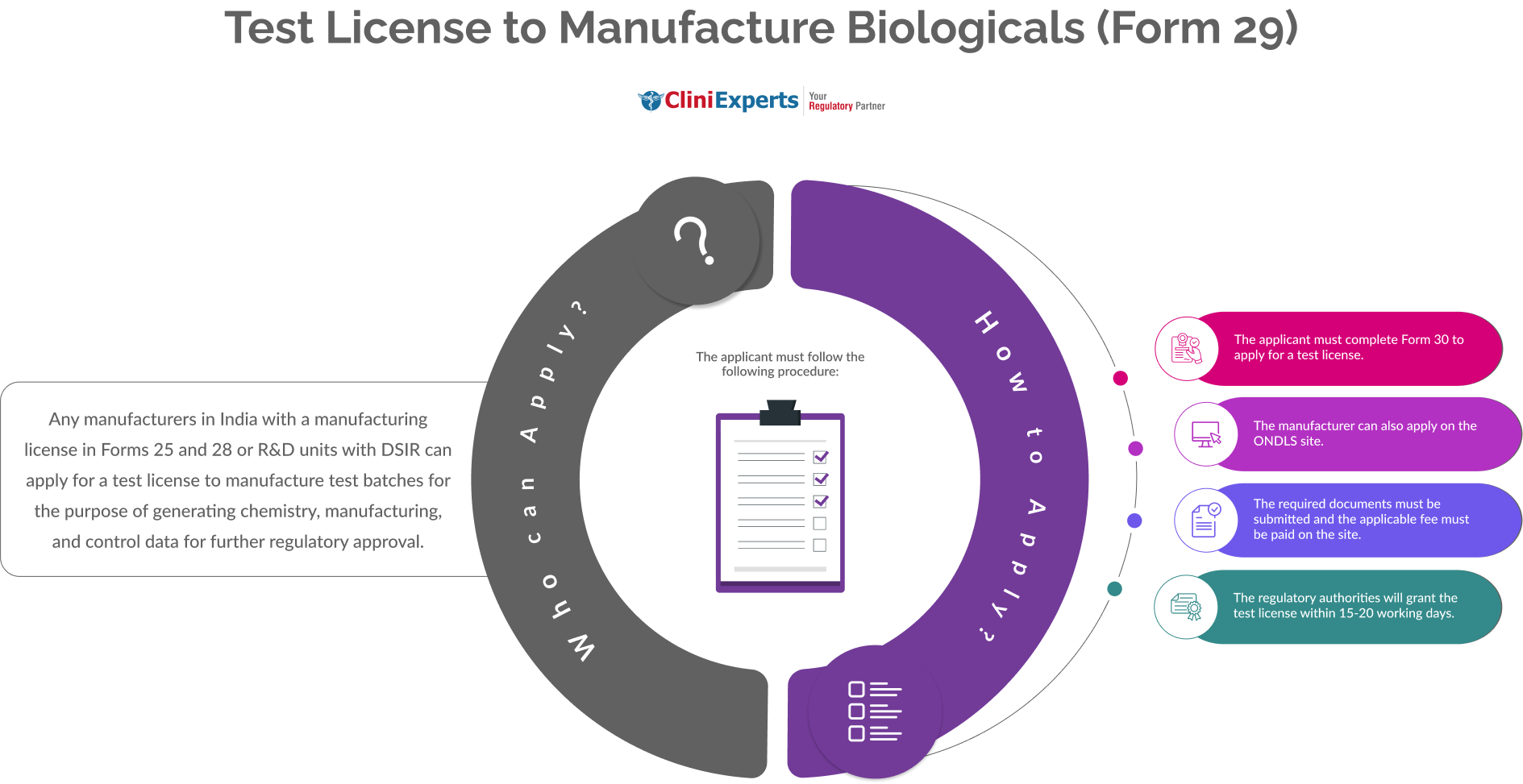
How To Apply?
The Applicant must follow the following process:
-

The applicant must complete Form 30 to apply for a test license.
-

The applicant must obtain Test License in Form CT-10 from CDSCO.
-

The manufacturer can apply on the ONDLS site or in Hardcopy.
-

The required documents must be submitted and the applicable fee must be paid on the site.
-

The regulatory authorities will grant the test license within 20-30 working days.

Validity
The test license would be valid for three years from the date of issue, unless suspended or canceled by the DCGI and/or SLA.
Fee Involved
Applicants who intend to apply must pay INR 500 per product during the application procedure.Important Documents

- If the firm has a manufacturing license on Forms 25 or 28 (License Application)
- Filled form 30
- Challan
- A copy of the manufacturing license or a copy of the Department of Scientific and Industrial Research (DSIR) certificate
- Other relevant documents
- If the firm does not have a manufacturing license
-
- Land Registration Certificate
- Partnership Deed / Article of Association
- Owner's ID Proof Technical Staff Documents
- NOC for fire and pollution
- Layouts
- Feed Challans
- Forms 25 and 28.
Timeline to get
Form 29
from Central Drugs Standard Control Organisation
20-30
Working DaysEssential Tips
- A precise justification of the sample quantity that will be manufactured should be presented.
- The documentation must mention all the tests and sample quantities that will be used for testing purposes.
Expert Advise
According to our experts, manufactures must ensure the following to avoid any delays or cancellation of your license.
- A clear justification of the sample quantity to be manufactured must be provided.
- All the tests and sample quantities that will be used for testing purposes should be mentioned in the documentation.
- Documents should be properly signed.
Frequently Asked Questions
Can the Form 29 license be renewed?
Yes, there is a provision for the renewal of Form 29 license for a period of one year at a time.

Who is the regulatory authority for Form 30?
The State Licensing Authority (SLA) is the concerned authority for approval of Form 30.

What are the conditions to be met to obtain a Form 29 license?
Applicants should use the drugs manufactured under the license exclusively at the place mentioned in the license. Further, the applicant should allow any appointed Drug Inspectors to enter the premises where the drugs are manufactured to ensure only examination, analysis or test work is being conducted. The manufacturer should also keep a record of the quantity of drugs manufactured and should provide any names of persons to whom the drugs have been supplied. If any of these conditions are not met, your license may be canceled or suspended.

