End to End Services For
BIS Certification
Trust CliniEpxerts for fully compliant, seamless services
CliniExperts – Your Obvious Choice As BIS Regulatory Partner
Streamline regulatory compliance with CliniExperts. We offer BIS certificate, CRS, and modification services. Our personalized solutions, streamlined process, and timely results ensure hassle-free BIS Registration compliances. Contact us today for efficient BIS regulatory consulting tailored to your needs.
About BIS?
Since 1987, the Bureau of Indian Standards or BIS has been fostering a culture of quality, reliability, and safety amongst a plethora of Indian products. Thanks to this apex standardization body, consumers are assured of product integrity and high standards for safe and reliable consumption. The governing body of BIS is inclusive and diverse, consisting 25 members from the federal and state governments, MPs, professionals from different domains, industry representatives, scientific and research firms, consumer organisations, and the like. The central minister of consumer affairs and its minister of state preside as BIS’ president and vice-president, respectively. Apart from product certification, BIS regularly updates standards to retain their market relevance, nforcs conformity assessment schemes, recognizes and operates testing and analyses laboratories, undertakes, and other world standardization bodies.

Other Contributing Factors
Product Categories Associated


|
Food
|
|
Cosmetics
|

|
Medical devices
|

|
IVD's
|
Types of Services
For Importers
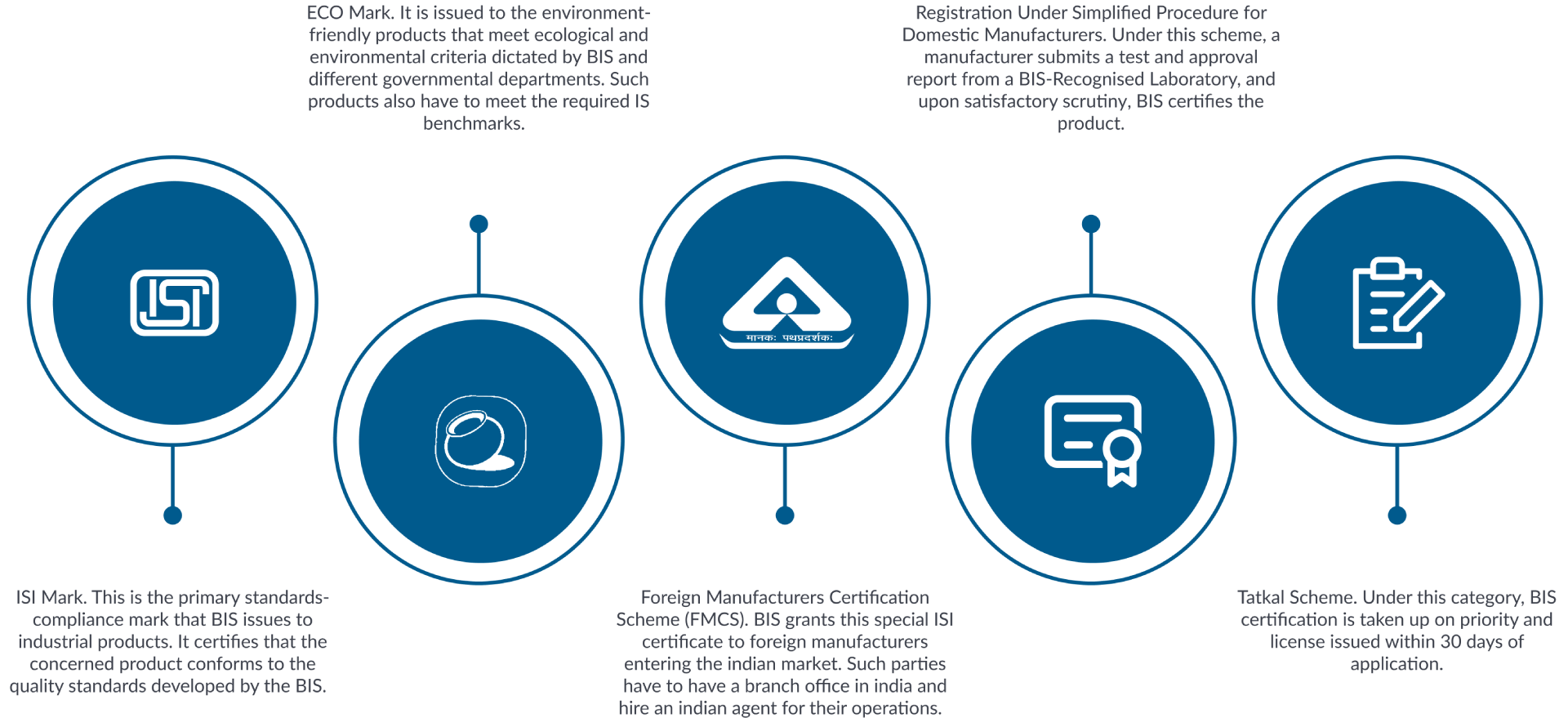
- BIS Certification for Foreign Manufacturer (ISI Mark)
- BIS CRS for Foreign Manufacturer for Electronic and IT Products
- Renewal of Certification for Foreign Manufacturer (ISI Mark)
- Modification and Amendment of Certification for Foreign Manufacturer (ISI Mark)
- Renewal of CRS for Foreign Manufacturer for Electronic and IT Products
- Inclusion of Series /endorsement of CRS for Foreign Manufacturer for Electronic and IT Products
- Modification and Amendment of CRS for Foreign Manufacturer for Electronic and IT Products
For Manufacturers
- BIS CRS for Domestic Manufacturer for Electronic and IT Products
- BIS Certification for Domestic Manufacturer (ISI Mark)
- Inclusion of Series /endorsement of Certification for Domestic Manufacturer (ISI Mark)
- Inclusion of Series /endorsement of Certification for Domestic Manufacturer (ISI Mark)
- Inclusion of Series /endorsement of CRS for Domestic Manufacturer for Electronic and IT Products
- Renewal of Certification for Domestic Manufacturer (ISI Mark)
- Modification and Amendment of Certification for Domestic Manufacturer (ISI Mark)
- Modification and Amendment of CRS for Domestic Manufacturer for Electronic and IT Products
Why Choose CliniExperts?
For over 13 years, we have been providing full-spectrum services for all aspects covering healthcare regulatory and compliance domains in India. Hence, when you entrust us as your BIS partner, you get:
- Services trusted by our customers
- Top-notch talent and experienced staff to handle your case
- Timely and satisfactory services
- Fully compliant, market-ready licensing and registration
- 24/7 response while your case is under process
- Deep understanding and knowledge of all licensing processes; hence error-free completion
- Resourcefulness – You can depend upon us for efficient and effective handling of your case throughout the BIS licensing funnel.

Testimonials
The Brand That Promises To Turn, Your Business Around!
Atomy India Pvt ltd
We have been associated with CliniExperts for about a year now for our food products related services for FSSAI compliances and licenses from CliniExperts and are completely satisfied with their performance. Quick turnaround time in responding to queries, meticulous planning in completing the task, alongside the extensive knowledge of the regulations of the entire team is highly appreciable and marks the professionalism and quality driven ethics of the organization. We highly recommend their services as we believe it is best in the industry so far.

Rajeev Rattava
Finance Manager
Morepen Laboratories Ltd.
The team at CliniExperts is proficient in the subject matter and provide prompt services . We are satisfied with our engagement and would recommend their services.

Anubhav Suri
Business Head
Proficon Medisol Pvt. Ltd.
We have been professionally associated with Cliniexperts for more than 5 years now. They have smoothly sailed us all these years in different projects & this has been possible because of the knowledge & dedication cliniexpert team has in their portfolio. All the best Cliniexpert. Keep going.

Ajitesh Trehan
Director
Frequently Asked Questions
Which products require registration?
The manufacturer must register their products which come under the Compulsory Registration Scheme (CRS) category as per the Bureau of Indian Standards (BIS) guidelines.

Are products which are tested from laboratories excluding the Bureau of Indian Standards (BIS) - approved laboratories acceptable?
No, products which require the grant letter with unique R – number and IS standard under the Compulsory Registration Scheme (CRS) category must be tested from Bureau of Indian Standards (BIS) - approved laboratories.

Is the Bureau of Indian Standards (BIS) license mandatory to import products to India?
Generally, the certification scheme of the Bureau of Indian Standards (BIS) is voluntary in nature. However, some products (notified by the Government of India under Quality Control Orders)can be imported to India provided they have an Indian Standard (IS) mark logo and a valid license from the Bureau of Indian Standards (BIS).

Will the IS Standard mark logo be present on the product or its packaging?
The IS Standard mark logo will be placed on both the product and its packaging. - However, if it is not feasible to place the IS standard mark logo on the product (due to size constraints), it can only be placed on its packaging. - For products with a display screen, provisions for E - labelling are also available.

Is nomination of the Authorized Indian Representative (AIR) mandatory?
Yes, the foreign manufacturer must nominate an Authorized Indian Representative (AIR).

Can product samples drawn during inspection be tested in laboratories approved by the International Laboratory Accreditation Cooperation (ILAC)/Asia Pacific Laboratory Accreditation Cooperation (APLAC)/government-owned laboratories in the country of the manufacturer?
No, product samples drawn out during inspection must only be tested in laboratories approved by the Bureau of Indian Standards (BIS). The applicant must send the product samples drawn by the Bureau of Indian Standards (BIS) to the laboratory. The product testing charges must be borne by the applicant.

