Cosmetic
Registration India | Import License, COS-1 & COS-2 Process
ACCELERATE YOUR COSMETIC
PRODUCTS TO INDIAN MARKET
CliniExperts provides comprehensive support for Cosmetic Registration in India, assisting importers and manufacturers in obtaining the Cosmetic Import License- COS 2 by CDSCO
Registration/Cos2 Registration Holder Compliance and Claims
Trusted By


























Many More
Fresh Registration (Cos-2) – Overview
The Central Drugs Standard Control Organisation (CDSCO) is the Indian regulatory body that issues Import Licenses for cosmetic items. The applicant or manufacturer must complete out form COS-2 to Import a cosmetic product into India for marketing purposes. The applicant is first required to register on the Sugam online portal. After Registration, the necessary documents should be uploaded, and fees should be paid. Following the evaluation of the application, CDSCO will either make a request for additional papers or issue the Import Registration certificate.

What is Form COS-1 and COS- 2
COS-1:
The CDSCO grants license to import and market cosmetics in India through this form.
COS-2:
This is the Import Registration Certificate issued by the CDSCO after the successful review and verification of the Form COS-1 application.
Who Can Apply - Cosmetic Registration Certificate
( COS- 2 ) in India ?
An application for registration of a cosmetic product intended to be imported into India shall be made through the online portal of the Central Government in Form COS-1 either by the manufacturer himself or by his authorised agent or the importer in India or by the subsidiary in India authorised by the manufacturer.
India Regulatory Expert Series
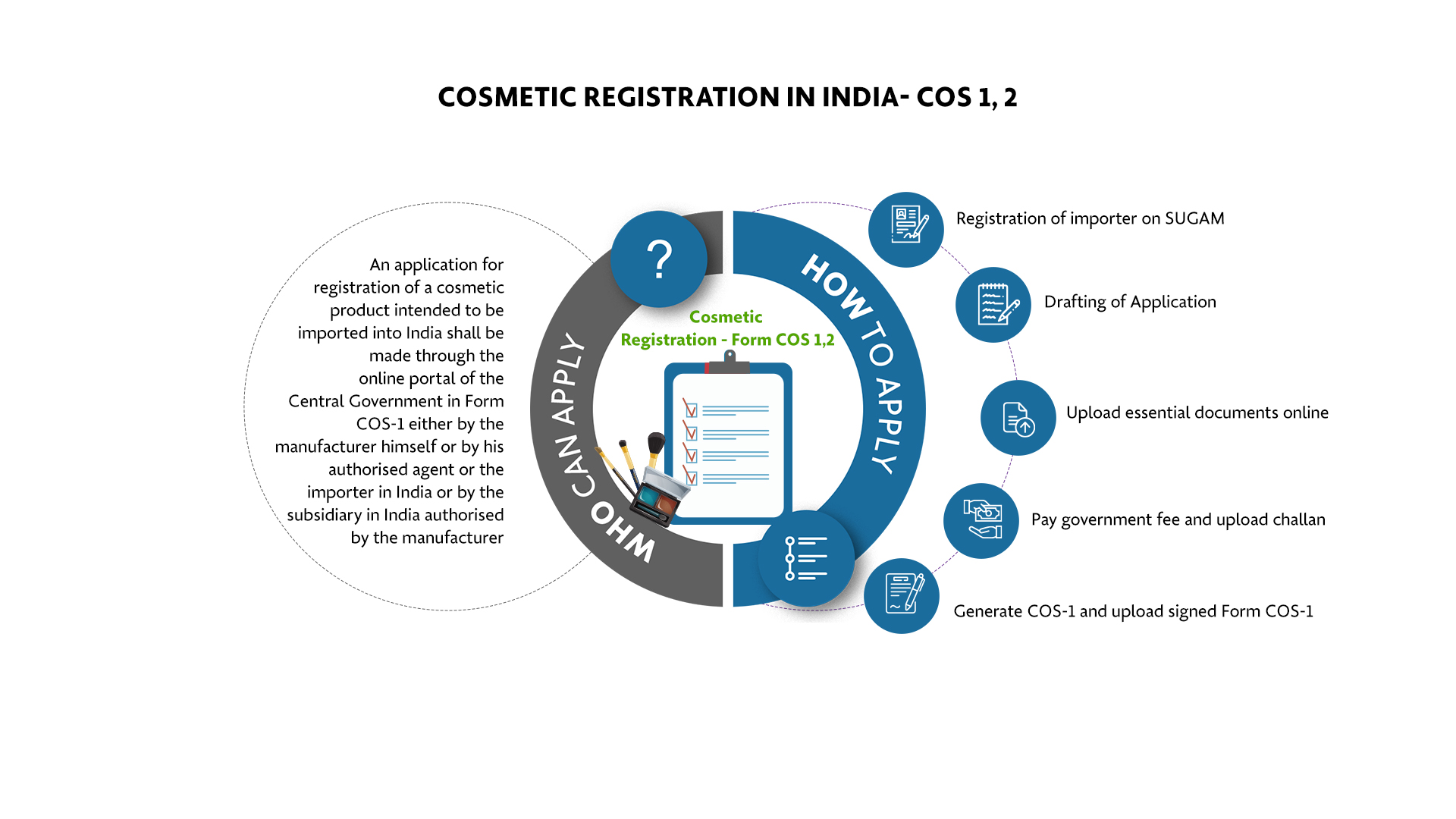
Step-by-Step Process for Cosmetic Registration in India - COS-1 & COS-2
 |
Registration of importer on SUGAM |
 |
Document compilation for application filing |
 |
Upload essential documents online |
 |
Pay government fee and upload challan |
 |
Generate COS-1 and upload signed Form COS-1 |
The Process Of Registration Is As Follows:
Cos1
- It is a predefined Performa by CDSCO to be filled to obtain Registration certificate.
- Cos1 can be filled online using the “Sugam” portal.
- It includes details about the premises of manufacturing, description of cosmetic products, brand name, pack size and their variants.
- Cos1 is accompanied by several documents.
Submitted
- Cos1(Duly signed and stamped) with other relevant documents are submitted to Drugs Controller General (I), CDSCO, New Delhi.
- Challan indicating the amount paid in INR along with the details of the importer is submitted in support of the application.
Registered (Cos2)
- Cos2 is also called as the Registration Certificate.
- The Registration Certificate shall be produced by the authorised importer/distributer/Indian authorised agent as and when required by the licensing authority.
- The timeline for approval is now 180 working days as per the new Cosmetic Rules, 2020.
- COS-2 is valid for five years.
- The government fee is 1000 USD per category, 50 USD per variant, and 500 USD per manufacturing site

Fees Involved
To obtain an import registration certificate for the import and market of cosmetic products in India, a fee of 1000 USD is charged per category, 50 USD per variants, and 500 USD per manufacturing site.

Validity
The validity of this license is five years.

Timeline to get Form COS- 2 from Central Drugs Standard Control Organisation
The Government timeline for Cosmetic Registration is 180 days.
Important Documents

While making an online application for obtaining a Grant license for selling products, the applicant must upload the following documents:
- Authorization from manufacturer.
- Product formulation, product label, and the certificate of analysis
- Free Sales Certificate.
- Testing Method.
Timeline to get COS 2 from CDSCO
6 to 9
MONTHSEssential Tips
To obtain an import license for the sale and distribution of cosmetics in India, an importer must ensure the following:
The validity of all legal documents must be checked.
The applicant must ensure consistency in the product names and names & addresses of manufacturers and importers across all documents.
All documentation must be in the English language.
The applicant must also provide other valuable information like heavy metal declaration and label claims of the product. In addition, the specifications of the cosmetic product must be as per the ninth schedule mentioned in the Bureau of Indian Standards.
Expert Advise
The categorization of the cosmetic products must be as per their intended use.
The applicant must ensure that the product-related and other details shared are consistent across all the documents.
The labeling of the cosmetic product should be as per the rules of the CDSCO. Also, the label should not contain any non-cosmetic claims.
Compliance And Analysis For Evaluation
While registering as a cosmetics supplier in India, you need to ensure that your product is identified under the right categories, with the right components, and is compliant with regulations.
We can help you with product analysis, compliance, and categorization to ensure an easy and successful registration.
PRODUCT AND INGREDIENT ANALYSIS
The content of your product needs to comply with the Bureau of Indian Standards and Drugs & Cosmetic Rules, 1945. Specific guidelines are also available for inner and outer labelling, ingredients, and coloring. CliniExperts runs a check on all these requirements when analysing your product to ensure that it fits Indian regulatory guidelines.
PRODUCT CATEGORIZATION
Cosmetic as well as personal care products cover a wide range of categories. These are defined on the basis of the intended use of the product, the region where the product is applied and so on. The regulatory authority in India, has prepared the guidelines for import and registration in which they have laid down roughly 80 categories for cosmetic products. These range from skin care products, oral hygiene products, hair care products and nail care products etc
COMPLIANCE
Your product and all your documents should comply in every way to the regulatory guidelines. You need to comply with the labelling guidelines given by CDSCO as per the registered category of your product. We help you through the compliance procedures and ensure that you miss nothing when registering your product for exporting to India.
Authorized Agent Support/ Registration Holder
CliniExperts can act as an authorised agent and can represent your organization as your Authorized Indian Representative/ Agent in accordance to Indian Regulatory Requirements without any commercial conflict of interest activities like product distribution, product marketing, product sales etc.
Distributor Identification
A successful product launch needs the right distribution channel that connects you to customer. We help you identify ideal distributors to create the right entry channel for your cosmetics in India also identify an Indian E-commerce partner & establish a partnership to open popular platforms to reach your customers.
Market Research And Price Research
By 2025, the Indian cosmetics market would grow to $20 billion. Our research services will help you understand Indian consumer preferences and guide you in choosing the right pricing strategy to appeal Indian buyers.
Joint Venture With Indian Manufacturers
It is always wise to have a local player associated with you when taking an entry into a new market. Taking the entry path through a joint venture can give you several benefits such as easy access to the Indian distribution networks.
Credentials
Registered Countries
Products Registered
Product Types
Brand Registered
Realted Blogs
Authorized Agent Support Services – Indian Authorised Representative Or Registration Holder Support

Related Service
AUTHORIZED AGENT/REGISTRATION HOLDER SUPPORT
Importer | Regulatory Body: CDSCO
CliniExperts has expertise starting from documentation to marketing of cosmetics. The seasoned team can guide your company to reach its complete growth potential by identifying appropriate distributor channels and e-commerce platforms. The strategies of the CliniExperts team
ASSISTANCE ON COSMETICS LABELLING
Importer | Regulatory Body: CDSCO
With a boom in the cosmetic market, it is essential to make your product such that it stands out from other competitors. A cosmetic product is also assessed by its consumers based on its label. Hence, a label should be appealing in a manner as well as per the regulations prescribed by the New Cosmetics Rules 2020 for quick approval from authorities.
PERMISSION FOR IMPORT OR MANUFACTURE OF NEW COSMETIC (FORM COS-12, 3)
Importer | Regulatory Body: CDSCO
The Central Drugs Standard Control Organization (CDSCO) issues import licenses for cosmetic products. The Cosmetic Rules, 2020 state that if a cosmetic product contains a novel ingredient, approval to import or manufacture it must be sought.


