Permission To Import / Manufacture New Cosmetics in India - COS 12 & COS 3

Get professional assistance to import or manufacture new cosmetic products in India as per Cosmetic Rule, 2020.
Permission To Import Manufacture New Cosmetics – Overview

Who Can Apply?
Indian Agent on the behalf of foreign / local cosmetic manufacturer having valid manufacturing license in Form 32/32A or COS-8/9.
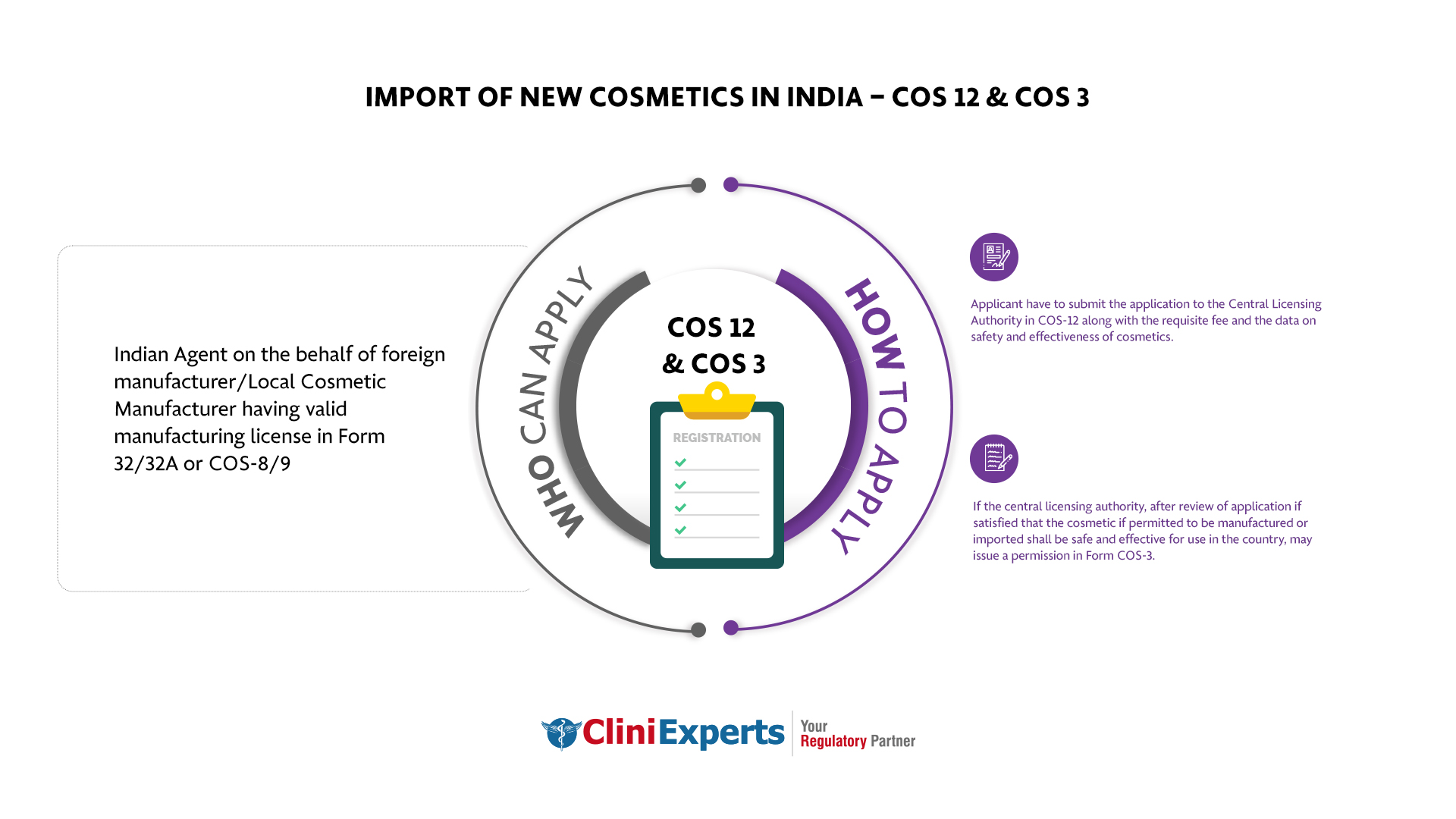
How To Apply?
The Applicant must follow the following process:
-

Applicant have to submit the application to the CLA in COS-12 along with the requisite fee and data on safety and effectiveness of cosmetics.
-

On reviewing the application, if the CLA finds the cosmetic safe and effective for use in the country, they may give permission in Form COS-3. This allows for its manufacturing or importing.

Validity
The license's validity has yet to be specified in the rule.

Fee Involved
A fee of 500 USD is applicable to obtain a license for import and manufacture of cosmetic products containing novel ingredients in India.Important Documents

Following documents are required for successful application of import license for cosmetic products:
- Duly filled application in Form COS-12
- Safety and efficacy data of the cosmetics
- Required fee
Timeline to get
COS 3
from Central Drugs Standard Control Organisation
Essential Tips
To obtain an import license for new cosmetics in India, an applicant must ensure the following:
- It is necessary that the application is duly signed and stamped.
- The data on safety should come from published research articles.
- All product and formulation information should be consistent throughout all papers.
- The applicant must also gather information on the novel ingredients used. In addition, the novel ingredient’s safety must also be demonstrated to the regulatory agencies.
Expert Advise
The use of a novel component in the formulation should be safe.
The importer must have information about the ingredients used before finalizing a manufacturer.
Frequently Asked Questions
What is New Cosmetic?
A cosmetic which contain novel ingredient which has not been used anywhere in the world or is not recognized for use in cosmetics in any National and International literature.

Is prior permission required for import or manufacture of New Cosmetics in India?
Yes. Prior permission from the Central Licensing Authority is required for import or manufacture of New Cosmetics in India.

What is procedure for prior permission for import or manufacture of New Cosmetics in India?
The applicant shall obtain a prior permission in Form COS-3, by making an application (in offline mode) in Form COS-12 along with requisite fee and documents as provided in Chapter V of the Cosmetics Rules, 2020, from Central Licensing Authority before registration of import of new cosmetic in India

What is procedure for import or manufacture of New Cosmetics in India?
An application for import and manufacture under Chapter III and under Chapter IV respectively, of the Cosmetics Rules, 2020 shall be made to the Central Licensing Authority or the State Licensing Authority, as the case may be, along with the prior permission obtained in Form COS-3 from the Central Licensing Authority.

What is the fee for grant of permission for new cosmetics?
$500

