Test License to Import In-vitro Diagnostic in India - MD 16 & MD 17

Need a permission to import IVD products in India to demonstrate its performance? CliniExperts’ professionals have expertise and assist you in securing a medical devices test license for importers in Form MD 17 by CDSCO.
Permission For In Vitro Diagnostic Kits Test License (Form MD 16, 17) – Overview
As per the Central Drugs Standard Control Organization (CDSCO) new provisions, an importer can import a small quantity of Class A, Class B, Class C, or Class D in-vitro medical devices by obtaining a test license. The in-vitro medical devices imported should be used for tests, evaluation, demonstrations, or training. The test license is granted in Form MD17.
To obtain a test license, the applicant must do the following:
- The applicant must apply for the import license to the Central Licensing Authority in Form MD-16.
- Form 16 is available on an online portal.
- The applicant must pay the required fees and upload all the necessary documents.
- After going through the application and documents, the CDSCO grants test license in Form MD-17.
Who Can Apply?
Importer who wants to import small quantity of IVD’s for the purpose of clinical investigations or test or evaluation or demonstration or training.
How To Apply?
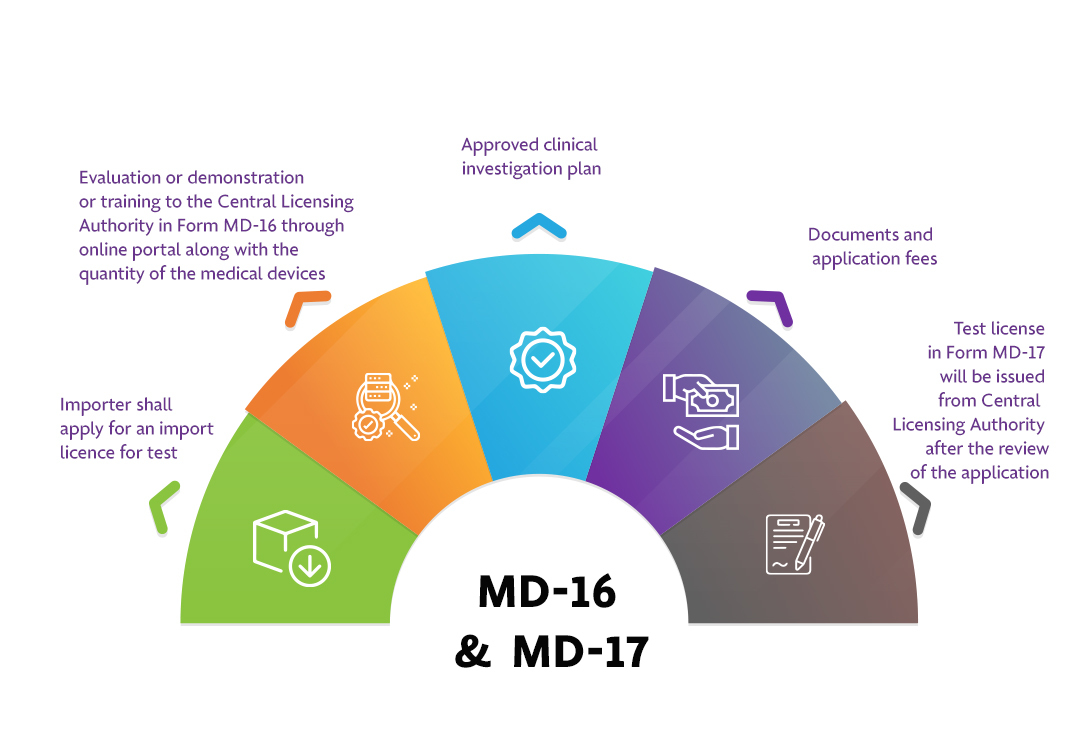
The Applicant must follow the following process:
-

Step 1: Importer shall apply for an import licence for test
-

Step 2: Evaluation or demonstration or training to the Central Licensing Authority in Form MD-16 through online portal along with the quantity of the medical devices
-

Step 3: Documents and application fees
-

Step 4: Test license in Form MD-17 will be issued from Central Licensing Authority after the review of the application.

Validity
3 Years

Fee Involved
The government fees is 100 USD for each distinct medical deviceImportant Documents

- Justification on quantity to be imported
- Brief description of the in vitro diagnostic kit including intended use, material of construction, design -Label and IFU
Timeline to get
MD 17
from Central Drugs Standard Control Organisation
30
WORKING DAYSEssential Tips
Get clarity on the regulatory status of your IVD products with CliniExperts professional assistance
- Quantity to be imported – This must be justified and clarified during submission preparation
- Name of place for testing/evaluation/demonstration/training- This is must requirement to be finalized during submission preparation.
Expert Advise
The holder of the test license shall maintain record of the activities undertaken including the name of manufacturer, quantity imported and date of import.
The consignment of in-vitro diagnostic medical device shall be accompanied by an invoice or statement showing the name and quantity of in-vitro diagnostic medical device.
The Test license must be exclusively used for the purpose for which it has been obtained.
Frequently Asked Questions
What is the sample size required to conduct performance evaluation of IVDs of Class B, Class C & Class D in the designated medical device testing labs?
The sample size shall be statistically significant as per the protocol designed and approved by respective Medical Device Testing Labs.

In case where the medical device is required to be taken to any place other than the ones mentioned in the test licence, how this can be done
In such case, the Central Licensing Authority shall be informed in writing before doing so.

A licencee, whose licence has been cancelled by Central licensing authority, when can the licencee appeal to authority
Within 45 days from date of such order.

Will products such as microbiological culture media, stains indicators and reagents used for food and water testing and is not intended to be used in human or animals for diagnosis of any disease or disorder be regulated under MDR, 2017?
No.

How many batches have to be evaluated for the submission of Performance evaluation reports for Class B, class- C & class- D IVDs?
The applicant shall submit performance evaluation reports (PER) for three independent batches of IVDs, manufactured by using three different lots of key raw materials (e.g., Antigen, antibody)

