Clarification Letter / No Objection Certificate for In-Vitro Diagnostic Kit (IVD) in India - NOC

Unclear about the regulatory status of In-vitro Diagnostic Kits in India. Let CliniExperts’ professionals assist you for getting a Clarification Letter Or No Objection Certificate (NOC) for In-Vitro Diagnostic Kits (IVD) from Central Drugs Standard Control Organisation.
Clarification letter / NOC from CDSCO for In-Vitro Diagnostics – Overview
The Central Drugs Standard Control Organisation (CDSCO) is the Indian regulatory body that governs the safety, efficacy, and performance of medical devices. The CDSCO also provides clarity to importers and manufacturers regarding the regulatory status of the product. The medical device section of the CDSCO office must receive the application for a clarification NOC in hard copy.
Who Can Apply?
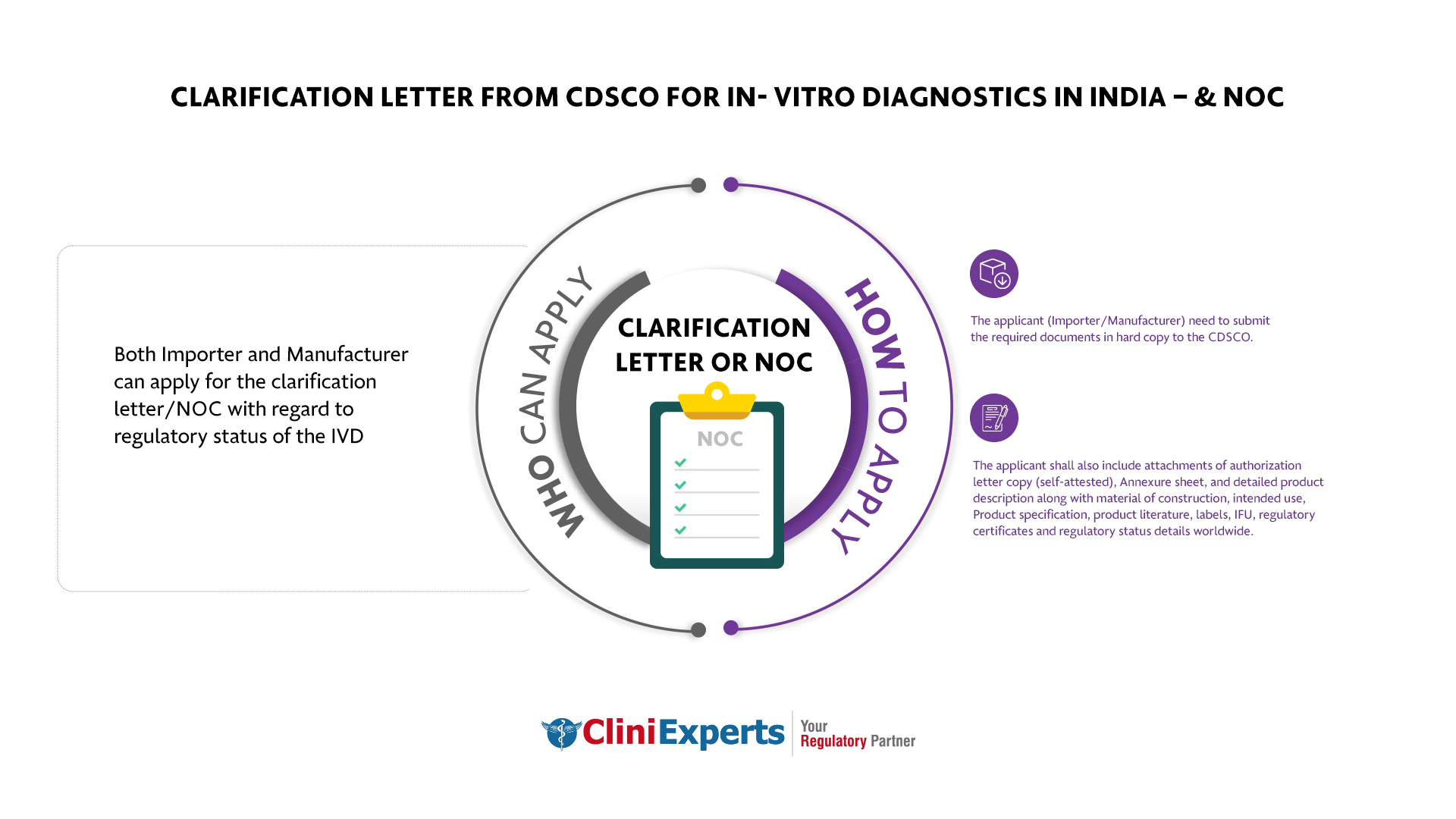
Both Importer and Manufacturer can apply for the clarification letter/NOC with regard to regulatory status of the IVD’s.
How To Apply?
The Applicant must follow the following process:
-

The applicant (Importer/Manufacturer) need to submit the required documents in hard copy to the CDSCO.
-

The applicant shall also include attachments of authorization letter copy (self-attested), Annexure sheet, detailed product description along with material of construction, intended use, Product specification, product literature, labels, IFU, regulatory certificates and regulatory status details worldwide.

Validity
Not Specified

Fee Involved
No fee is applicable for this serviceImportant Documents

The following documents are required when applying for a clarification NOC:
- Documents entailing the specifications or descriptions of the product
- Regulatory Certificate from country of origin
Timeline to get
NOC
from Central Drugs Standard Control Organisation
Essential Tips
Unclear about the regulatory status of In-vitro Diagnostic Kits in India. Let CliniExperts’ professionals assist you for getting a In-Vitro Diagnostic Kits (IVD) No Objection Certificate (NOC) or Clarification Letter from Central Drugs Standard Control Organisation.
- The applicant must ensure that the specifications of the products are clearly mentioned.
- Regulatory certificate from the country of origin is essential for the application.
- It may take approx. 30-45 days to process the application as the government has not specified the time frame.
Expert Advise
The exact intended use of the product, as stated in the IFU, must be specified by the applicant in the submission.
Frequently Asked Questions
What is clarification NOC?
It is a No Objection Certificate (NOC) or a clarification issued by the governing body that reflects the subjected product's regulatory status in India.

Who is eligible to submit an application for a Clarification NOC?
The application for a clarification NOC can be submitted by both importer and manufacturer.

