Registration For Non-Notified In Vitro Diagnostic in India

CliniExperts holds a valid Drug Wholesale License and provides 360-degree regulatory solutions to importers and manufacturers of IVDs and other medical devices to launch their products in the Indian market. The professional team at CliniExperts will guide you through all the paperwork and help acquire speedy approvals to enable a timely and successful launch of your product.
Registration For Non-Notified In Vitro Diagnostic – Overview
The Central Drugs Standard Control Organisation (CDSCO) oversees all medical devices and their approvals in India. All non-notified In-vitro Diagnostic (IVD) medical devices need registration with the CDSCO. This excludes those already under the notified category of medical devices.
Any Indian manufacturer or foreign importer of non-notified in-vitro diagnostics can use this service. They can apply for a registration number through this service.
The applicant must apply for registration through the identified online portal, SUGAM. It is maintained by the Ministry of Health and Family Welfare.
Who Can Apply?
Any foreign importer or a local manufacturer who wishes to acquire a Registration Number of non-notified in-vitro diagnostics (IVDs) can apply for this service.
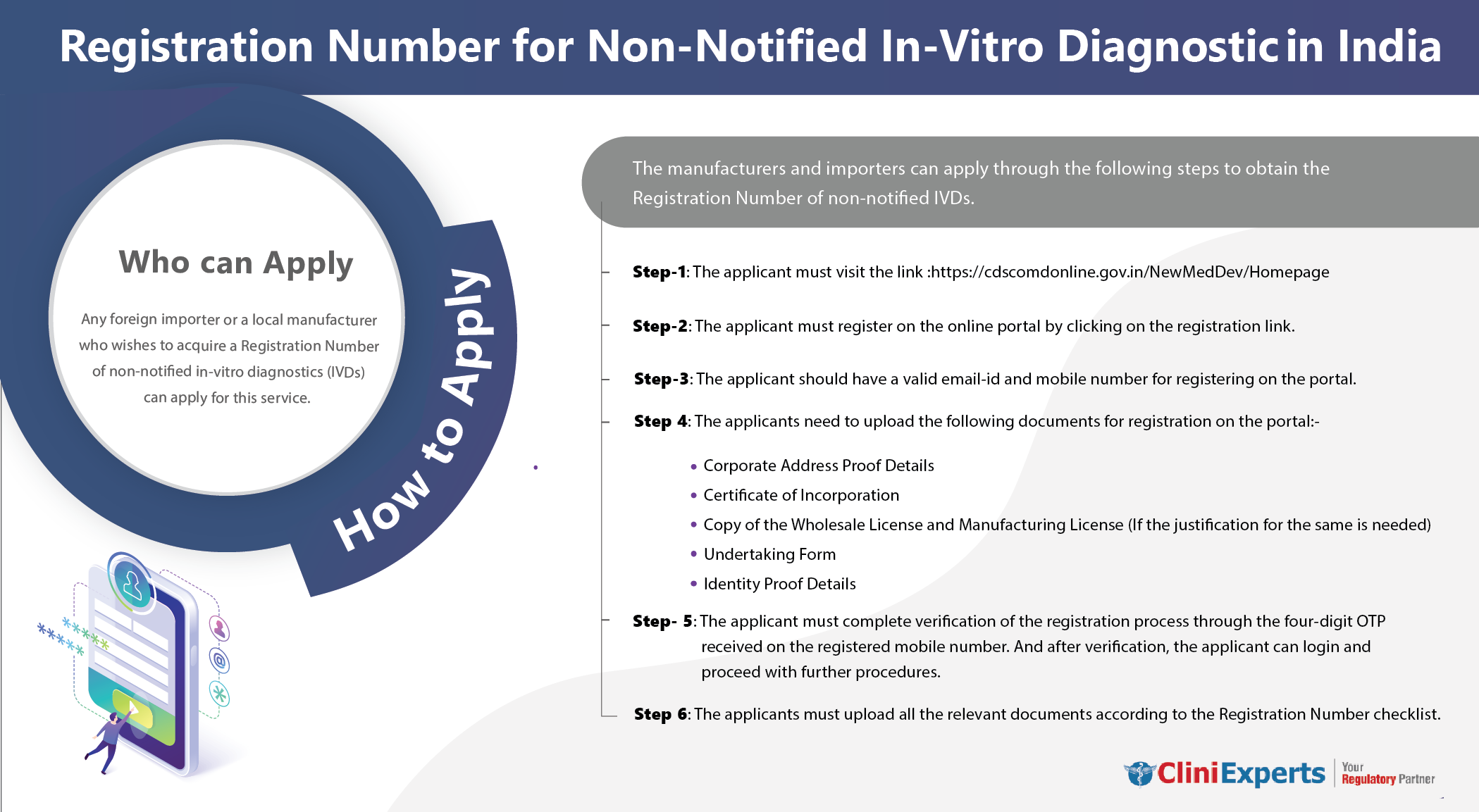
How To Apply?
The Applicant must follow the following process:
-

Step 1: The applicant must visit the link:https://cdscomdonline.gov.in/NewMedDev/Homepage
-

Step 2: The applicant must register on the online portal by clicking on the registration link.
-

Step 3: The applicant should have a valid email-id and mobile number for registering on the portal.
-

Step 4: The applicants need to upload the following documents for registration on the portal:
• Corporate Address Proof Details
• Certificate of Incorporation
• Copy of the Wholesale License and Manufacturing License (If the justification for the same is needed)
• Undertaking Form
• Identity Proof Details -

Step 5: The applicant must complete the registration process verification. This is done using the four-digit OTP received on the registered mobile number. After verification, the applicant can log in and proceed with further procedures.
-

Step 6: Applicants must upload all relevant documents. It must be according to the Registration Number checklist.

Validity
The Registration number for the non-notified in-vitro diagnostics (IVDs) licenses has a validity period.
- The license validity for Class A and B in-vitro diagnostic (IVDs) devices is till September 2022.
- The license validity for Class C and D in-vitro diagnostic (IVDs) devices is till September 2023.

Fee Involved
The Government fee is not applicable for obtaining a Registration number for the non-notified in-vitro diagnostics (IVDs).Important Documents

The essential documents required for this voluntary registration are:
- ISO 13485 Certificate
- In case of import, a Free Sale Certificate from the country of origin
- Device description including material of construction, intended use, shelf life, sterilization, dimension, component, or accessories
- Other important documents regarding the device as mentioned on SUGAM portal
Timeline to get from Central Drugs Standard Control Organisation
Essential Tips
CliniExperts has some essential tips that might benefit their clients:
- The applicant must have an ISO 13485 Certificate as a mandatory requirement for the application process. The applicant might face a problem if the product's regulatory status is unclear. Thus, the applicant must seek clarity from the authority before applying for the voluntary registration number. This step is essential.
- If the Authority has not classified the product, the applicant must finalize the product classification. This step is necessary before proceeding.
Expert Advise
The importer and the manufacturers should act in accordance with the safety and quality-related requirements.
Once the validity period of the Registration Number expires, the terms and conditions for the Import License and Manufacturing License are applicable.
The importer and the manufacturers should act in accordance with the safety and quality-related requirements.
Related Services
SUGAM Registration- MD/ IVD
Importer | Regulatory Body: CDSCO
The registration process includes several steps to obtain approvals. The process involves registration, undertaking, and uploading the documents, complying with the given rules and regulations. We at CliniExperts, make sure that our expert team creates your registration easy and quick so that you can focus on more important aspects of your product launch...
Test license to manufacture In Vitro Diagnostics in India – MD 12 & MD 13
Manufacturers | Regulatory Body: CDSCO
Registration for in-vitro diagnostic medical devices for manufacturing can be time-consuming and tedious; we at CliniExperts will take care of all the registration procedures while you focus energies on planning your product launch. From the application process forms to undertaking a license test, we assist in every registration aspect to help you smoothly start the manufacturing process.
Import License For In-Vitro Diagnostic Kits in India – Form MD14 & MD15
Importers | Regulatory Body: CDSCO
Get Your Import License for In-Vitro Diagnostic Kits (IVD) and Medical Devices with CliniExperts. Hassle-free assistance in providing Import License of Form MD 15.
Frequently Asked Questions
Is the ISO 13485 Certificate a mandatory requirement for a manufacturer or importer?
Yes. The ISO 13485 Certificate is a mandatory requirement for obtaining the Registration Number for the in-vitro diagnostic devices. Therefore, all the manufacturers or importers should have ISO 13485 Certificate before applying for this service.

Who can file the application for a Registration Number?
Any foreign importer or manufacturer in India can apply for a Voluntary Registration Number.

Where can we find more information on the risk class of non-notified devices?
To know more about the risk class of non-notified devices, you can refer to the CDSCO Classification List.

