Import License for Drugs in India - Form 8/ 8A & Form 10/ 10A

Do the complexities of the CDSCO's licensing process of importing biologicals to India leave you feeling overwhelmed? Partner with CliniExperts, your one-stop solution for securing a Form 10 Import License for drugs efficiently and smoothly. Our expert guidance with a streamlined and meticulous approach, and deep industry understanding ensures complete forms filing, minimizing the risk of delays or rejections.
Import License for Drugs – Overview
Who Can Apply?
Any Indian citizen holding a wholesale license or manufacturing license under the Drugs and Cosmetics Act and Rules is eligible to apply for a Biological Import License.
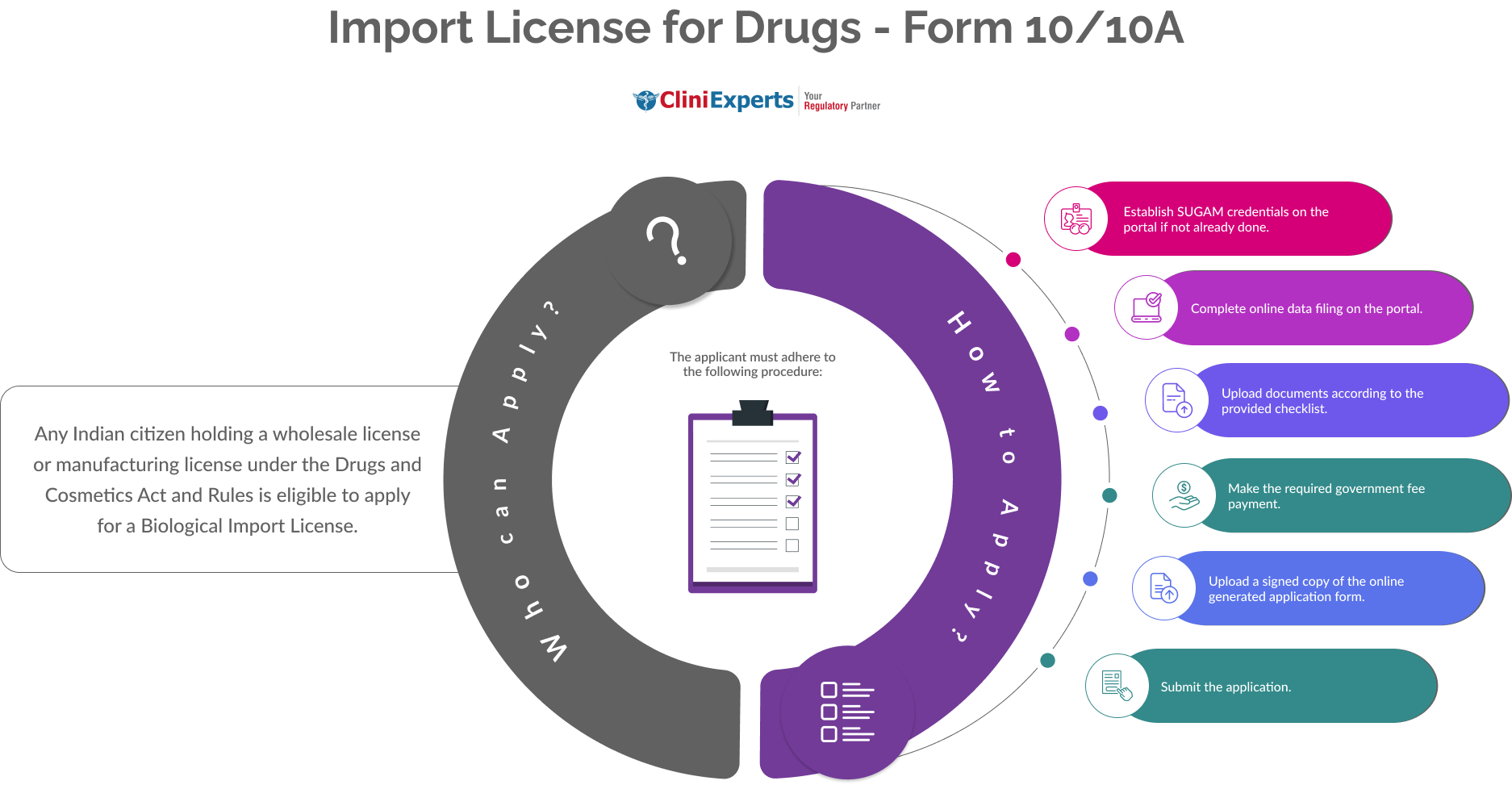
How To Apply?
The Applicant must follow the following process:
-

Establish SUGAM credentials on the portal if not already done.
-

Complete online data filing on the portal.
-

Upload documents according to the provided checklist.
-

Make the required government fee payment.
-

Upload a signed copy of the online generated application form.
-

Submit the application.

Validity
The biological import license will remain valid for three years from the date of approval. It will expire sooner if the Registration Certificate expires before the three-year period ends.
Fee Involved
To acquire a Biological Import License for importing r-DNA products, blood products, vaccines, or stem cells in India. The applicant must pay INR 10,000 for the first product. An additional fee of INR 1,000 is required for each additional product.Important Documents

- Registration Certificate (Form 41)
- Form 9
- For wholesalers: Wholesale license (Form 20B/21B/21C)
- For manufacturers: Manufacturing license in Form 25/28
- Labels (Primary & secondary)
Timeline to get
Form 10/ 10A
from Central Drugs Standard Control Organisation
45
DaysEssential Tips
- Compliance with the conditions outlined in the Covering Letter of the Registration Certificate is imperative. Failure to do so may result in queries or delays in approval.
- The importer's details like name and address must align with Form 9 and correspond with the details provided on the wholesale license.
- Artwork details should adhere to Rule 96 of The Drugs and Cosmetics Rules, 1945. Any deviation may lead to queries or delays in approval.
- All submitted documents must be properly stamped and signed.
Expert Advise
To acquire the permission to import biologicals into India, our experts advice to have the following in place:
- Form 9 with a signature by either an Indian Agent or a Manufacturer.
- The imported product must possess a shelf life exceeding 60% from the import date.
- Importers are prohibited from diverting drugs for sale if imported under Special Economic Zones. Note that a Biological Import license is unnecessary when ordering intermediates or inactive bulks.
Frequently Asked Questions
Who can apply for import license?
Any firm/company having wholesale license in Form 20B/21B/21C or manufacturer having manufacturing license in Form 25/28 can apply for import license.

Who can provide form 9?

Can importer import the products without getting registration certificate (Form 9)?
No, however if importer is other then registration certificate holder then undertaking in form 9 from Indian agent along with copy registration certificate would be required.

Is India specific label is mandatory requirement to get the Import license?
Yes.

Whether copy of new drug permission required for import license application?

What companies or manufacturers are eligible to apply for a Biological Import license for importing biologicals into India?

Is an India-specific label required for the company or manufacturer to obtain a biological import license?

Is it necessary for the company or manufacturer to provide a copy of the new drug permission when applying for a biological import license?

If an importer intends to import multiple drugs from various ports, do they need separate biological import licenses for drugs registered under a single Registration Certificate by a sole importer?

Is it possible for the company to apply for a Registration Certificate (Form 41) and a biological Import license (Form 10) simultaneously?

