Registration Certificate in India - Form 40 & Form 41

Elevate your pharmaceutical development with CliniExperts. We simplify the process of obtaining your Registration Certificate (Form 40,41) with expert guidance. Enhance your research and innovation capabilities with our support!
Registration Certificate – Overview
The registration certificate in Form 41, which is necessary for the registration of a manufacturing site and drug, must be obtained before applying for an import license. This requirement applies to both manufacturers and importers. The license can be obtained from the CDSCO. A brief overview of the entire process is given below:
To complete the application process:
- Form 40 must be submitted, accompanied by the specified documents listed in the checklist.
- Additionally, the government fee as stipulated in the Drugs and Cosmetics Act should also be paid.

Who Can Apply?
Individuals eligible to apply for the license include those who possess either a wholesale license (Form 20B and 21B) as importers or a drug manufacturing license.
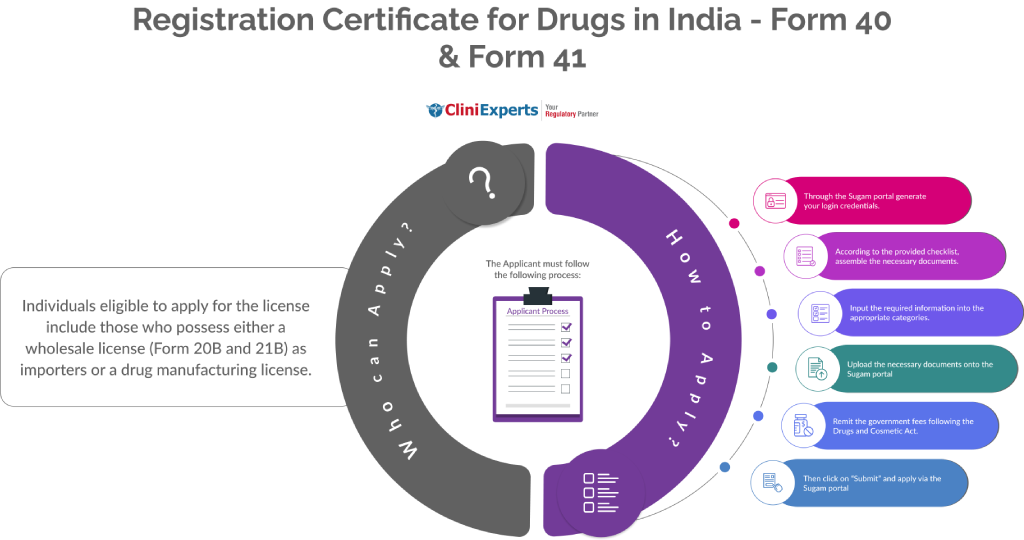
How To Apply?
The Applicant must follow the following process:
-

Through the Sugam portal generate your login credentials.
-

According to the provided checklist, assemble the necessary documents.
-

Input the required information into the appropriate categories.
-

Upload the necessary documents onto the Sugam portal.
-

Remit the government fees following the Drugs and Cosmetic Act.
-

Then click on “Submit” and apply via the Sugam portal.

Validity
The licensing process takes approximately nine months, and once issued, the license is valid for three years from the date of issuance.

Fee Involved
The license fee consists of USD 10,000 per site plus an additional USD 5,000 per drug.
Important Documents

The essential documents needed to acquire licenses are as follows:
- Manufacturing License
- A Free Sale Certificate.
- Form 20B and 21B for the wholesale license
- A Power of Attorney
- Notarized Site Master File
- Apostilled GMP Certificate
- CoPP
- Notarized Drug Master File
Timeline to get
Form 41
from Central Drugs Standard Control Organisation
9
MONTHSEssential Tips
These are some of the key prerequisites required when applying for the license:
- The necessary documents must be prepared following the provided checklist.
- A valid copy of either a wholesale license or a manufacturing license is essential.
- Registration of the company on the Sugam portal is a prerequisite
Expert Advise
Our expert guidance provided to clients, customers, or end-users regarding the licensing procedure:
Make sure to keep the Notarized Drug Master Files and Site Master Files handy.
Remember that the government fee is USD 10,000 per manufacturing site and USD 5,000 per manufacturing site.
Ensure to provide a valid copy of either a wholesale license or a manufacturing license.
Related Services
Manufacturing License in India
Manufacturer | Regulatory Body: CDSCO
Elevate your pharmaceutical development with CliniExperts. Get expert guidance on Manufacturing License Service with us. Boost your research and innovation with us!
New Drug Approval in India - Form 29 & Form 30
Manufacturers | Regulatory Body: CDSCO
CliniExperts is a 360-degree regulatory solutions provider with over a decade of industry regulatory experience. Our deep industry networks and highly updated team create a smooth experience in receiving necessary licenses and approvals for your products.
Wholesale Drug License in India - Form 20B 21B & Form 19
Manufacturer | Regulatory Body: CDSCO
CliniExperts offer strategic planning services for Wholesale Drug License to start with your pharmaceutical business and sell drugs at distributor level. Our hand in glove approach ensures that at no point you find yourself battling with any process by yourself.
Permission to Manufacture Class C & D In- Vitro Diagnostics in India
Manufacturers | Regulatory Body: CDSCO
CliniExperts, our team of experts is thoroughly updated with the latest requirements of the various authorities, enabling complete and meticulous documentation, timely application and quick approvals/licenses.
Frequently Asked Questions
What is the official timeframe set by the government to acquire the registration certificate?
The government's stipulated timeline for obtaining the registration certificate is 9 months, equivalent to 270 days.

Which documents need to undergo either apostillation or notarization?
The following documents require either apostillation or notarization: Manufacturing License, Power of Attorney, Free Sale Certificate, CoPP, Site Master File, GMP Certificate, and Drug Master File.

What is the main prerequisite for submitting the application?
The importer's company should have its registration completed on the Sugam portal.

Does the Form 41 registration certificate serve as a license for importing drugs?
No, an Import license in Form 10 will be necessary even after obtaining the registration certificate in Form 41.

What is the government fee for the registration of a manufacturing site and a product?
The fee is USD 10,000 for each site and USD 5,000 for each drug.

