Manufacture unapproved API for development of formulation for test or analysis or CT or BA or BE in India - CT-13 & CT-15

Cliniexperts, with over a decade of regulatory experience, facilitates smooth licensing processes. Our adept team specializes in securing approvals for unapproved Active Pharmaceutical Ingredients (API) following CT-15 guidelines. Trust us to navigate regulations efficiently, ensuring swift approvals for your products.
Manufacture unapproved API for development of formulation for test or analysis or CT or BA or BE – Overview
Who Can Apply?
Applicant having manufacturing license in form 25, form 28 or DSIR approval.
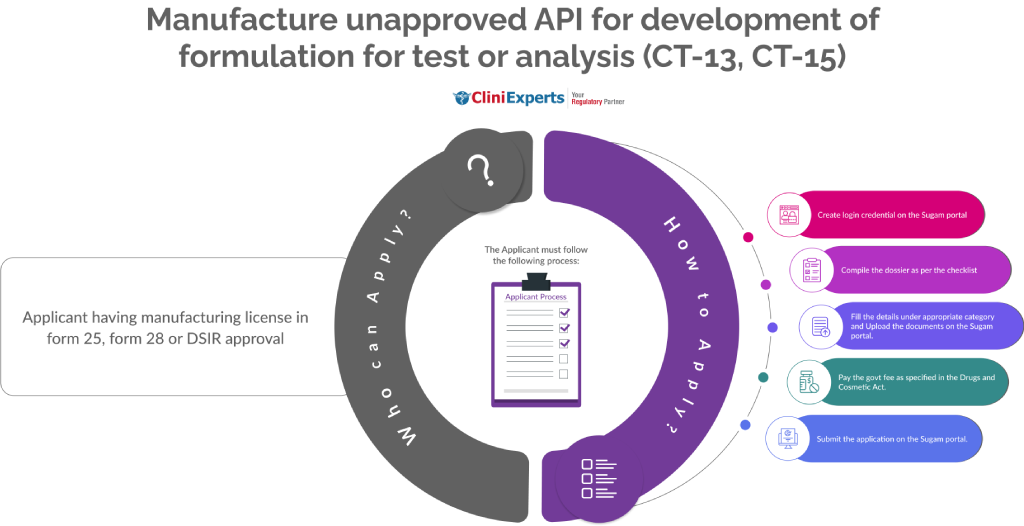
How To Apply?
The Applicant must follow the following process:
-

Create login credential on the Sugam portal.
-

Compile the dossier as per the checklist.
-

Fill the details under appropriate category and Upload the documents on the Sugam portal.
-

Pay the govt fee as specified in the Drugs and Cosmetic Act.
-

Submit the application on the Sugam portal.

Validity
The license is valid for 3 years.

Fee Involved
The government charges 5000 INR per drug for the license.Important Documents

The documents mentioned below should be present during the application process for CT-13:
- Manufacturing License (FORM 28 & FORM 25)
- Detailed utilization for each drug
- Proposed SOP of testing/analysis
- Proposed SOP for manufacturing
- List of manufacturing equipment
- Proposed specification/STP
Timeline to get
CT-15
from Central Drugs Standard Control Organisation
90
DaysEssential Tips
The primary considerations that need to be made while preparing and submitting this license application are:
- The company should be registered on the Sugam portal.
- Manufacturing license must be available with the manufacturer before applying.
- The dossier should be compiled as per the checklist.
- The obstacles or issues that are most likely to arise when filling out or applying are:
- Lack of a Valid manufacturing license at the time of application.
- Missing manufacturing process SOP.
- Missing proposed testing SOP.
- Lack of one or more of these documents can lead to rejection of application.
Expert Advise
According to the experts, clients should look out for the following during the license application procedure:
- Quantity breakup should be given with proper justification.
Related Services
Manufacturing License - Form 24, 24A, 24B, 24C, 24D, 24E, 24F & 25, 25A, 25B, 25C, 25D, 25E
Manufacturer | Regulatory Body: CDSCO
Elevate your pharmaceutical development with CliniExperts. Get expert guidance on Manufacturing License Service with us. Boost your research and innovation with us!
Frequently Asked Questions
What is the primary requirement for submission of an application?
The company must be registered on the Sugam portal before going for application.

What are the documents which are required?
The most important documents are the manufacturing license, quantity breakup, proposed manufacturing and testing SOP, list of analytical and manufacturing instruments to be used for manufacturing.

What is the govt fee for permission in CT 15?
5000 INR per drug is the government fees to be paid of Sugam portal while submitting application form.

What is the government timeline to obtain PERMISSION?

Can a manufacturer formulate a product of an unapproved API after getting permission on CT 15?
No. After obtaining permission on CT 15 one needs to obtain the permission on CT 14 for the formulation.

