FOOD
Navigating Functional Food Supplements: A Comprehensive Guide for Importers
Empowering Importers with Insights and Strategies for Functional Food Supplement import
CliniExperts - Regulatory Excellence Made Easy
Are you navigating the complex landscape of regulatory compliance for your food supplements? Look no further! We offer a complete suite of services to guide you from project inception to completion. Our team provides invaluable regulatory manpower support, leveraging years of expertise in food product scientific details and lab testing parameters. From preparing critical regulatory documents to offering guidance on clinical trial objectives and parameters, we've got you covered. Additionally, we assist in selecting appropriate testing labs, ensuring thorough and accurate compliance every step of the way. With us by your side, you can navigate regulatory hurdles with confidence and efficiency.
FSSAI – How It Regulates Food Businesses
The Food Safety and Standards Authority of India (FSSAI) is a statutory body under the administration of the Ministry of Health and Family Welfare, Government of India.
Its primary role is to regulate the manufacture, storage, distribution, sale, and import of food articles, ensuring their safety and quality. FSSAI establishes science-based standards for food products, aiming to provide safe and wholesome food for human consumption. CliniExperts has the experience and expertise to fulfil the entire regulatory process with a zero-defect capability.
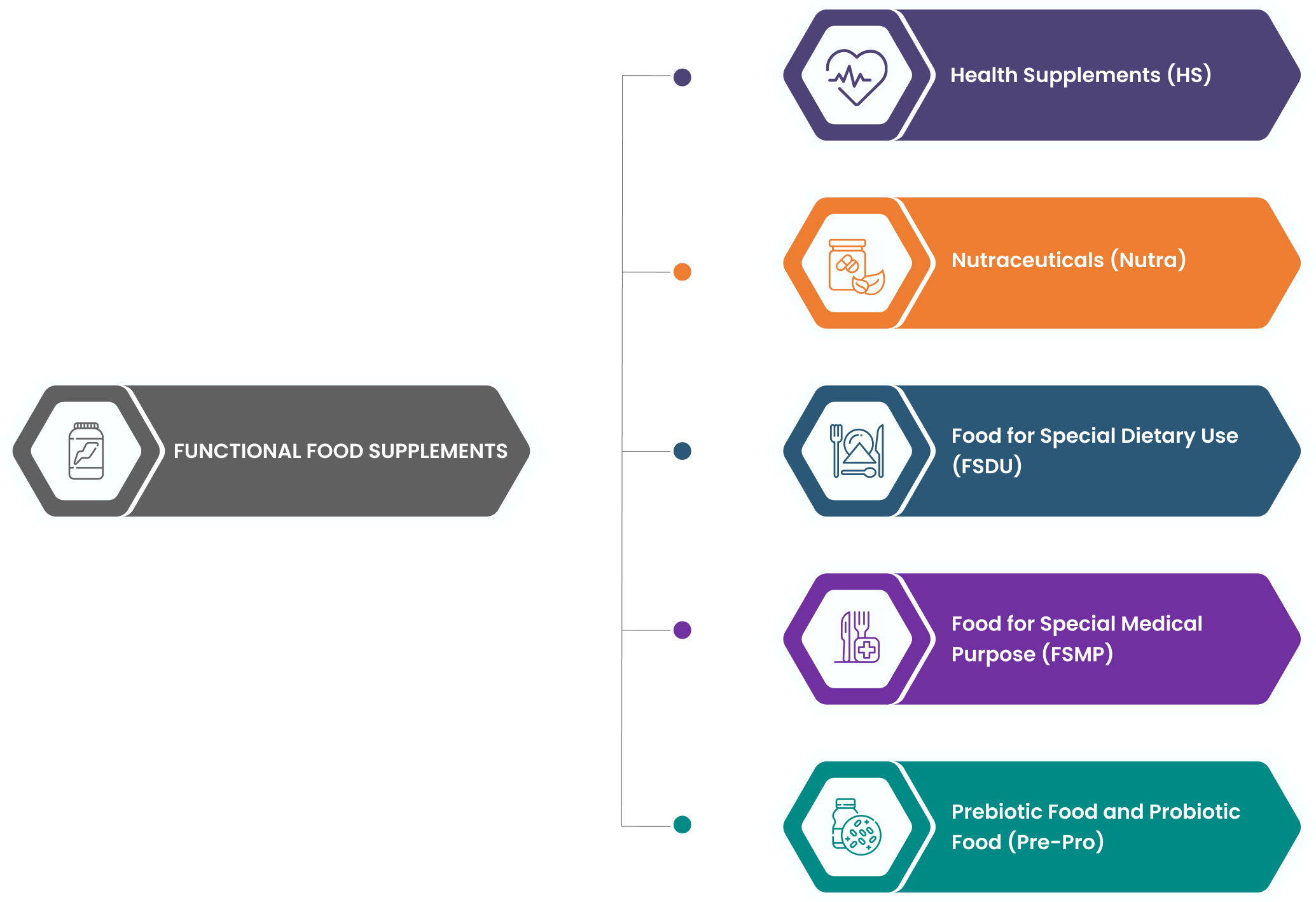
About Functional Food
- Functional foods are ingredients that offer health benefits beyond their nutritional value.
- Some types of functional foods contain supplements or other additional ingredients designed to improve health.
- They are specially processed or formulated for specific nutritional or dietary purpose and are different from foods intended for normal consumption.
- They are meant to be consumed orally in defined quantities and duration and shall not include products intended for parenteral use.
- Health Supplements ( HS)
- Nutraceuticals (Nutra)
- Food for Special Dietary Use (FSDU)
- Food for Special Medical Purpose (FSMP)
- Prebiotic Food and Probiotic Food (Pre-Pro)
Health Supplement (HS)
It is a category of foods, which consists of a concentrated source of nutrients (like proteins, minerals, vitamins, amino acids) and/or other ingredients with nutritional or physiological effects, singly or in combination, whose purpose is to supplement the normal diet.


Nutraceuticals (Nutra)
It is a category of foods which consists of extracts, isolates and purified chemical compounds having a physiological benefit and help to maintain health.


Food for Special Dietary Use (FSDU)
It is a category of foods, which are specially processed or formulated to satisfy particular dietary requirements which exist because of a particular physical or physiological condition and/or specific diseases and disorders and which are presented as such. The composition of these foodstuffs must differ significantly from the composition of ordinary foods of comparable nature, if such ordinary foods exist. FSDU which are intended to be used as an adjunct for the management of diseases/disorders only under medical prescription and supervision shall normally be categorized under FSMP.


Food for Special Medical Purpose (FSMP)
It is a category of foods for special medical uses, which are specially processed or formulated and presented for the dietary management of patients and may be used only under medical supervision. They are intended for the exclusive or partial feeding of patients with limited or impaired capacity to take, digest, absorb or metabolize ordinary foodstuffs or certain nutrients contained therein, or who have other special medically-determined nutrient requirements, whose dietary management cannot be achieved only by modification of the normal diet, by other foods for special dietary uses, or by a combination of the two.


Prebiotic and Probiotic Food (Pre-Pro)
Prebiotic food means food that contains added ingredients which are non-viable food components that confer health benefits to the consumer by modulation of gut microbiota.
Probiotic food means food with live micro-organisms beneficial to human health, which when ingested in adequate numbers as a single strain or as a combination of cultures, confer one or more specified or demonstrated health benefits in human beings.

Why Choose CliniExperts?
Embrace CliniExpert's trusted guidance to capitalize on the thriving opportunities in importing functional food supplements.
Contact our team to get the best support for regulatory services in India.


How CliniExperts Supports Clients?
Clients primarily choose us for four key reasons:
Our client roster is steadily growing, and we boast a 100% retention rate. When seeking a regulatory partner, market stature and size are crucial factors, and CliniExperts excels in this regard. Ethics, integrity, and excellence are at the core of our values. We are known for our responsible and respected approach, coupled with pragmatism and ample resources to navigate regulatory complexities. Our teams are composed of industry frontrunners who demonstrate exceptional talent and unwavering commitment to quality across all functions. We take pride in our up-to-date knowledge and information pool, ensuring that our applications are perfectly compliant in every aspect. Together, these attributes often earn us the esteemed status of “Most Favored Service Provider." Let’s join hands for a mutually rewarding and enduring association!Form Names: NA
Food Product Compliance For Food Supplements
Ensuring food product compliance is crucial to guarantee that products manufactured meet FSSAI norms comprehensively. Compliance involves adhering to all standards and regulations to avoid penalties or legal repercussions for the Food Business Operator (FBO). Maintaining compliance not only mitigates risks but also fosters the establishment of a trustworthy brand.
Regulatory Body Requirement
FSSAI Food Product compliance ensures that products adhere to the essential requirements set forth by FSSAI through directives, standards, and regulations. It applies to manufacturers, marketers, sellers, and FBOs involved in storing and distributing food supplements and nutraceuticals. Compliance with FSSAI Product regulations is overseen by FSSAI and related bodies like Legal Metrology, where applicable. The service aims to verify that product formulations and labels align with FSSAI standards, involving an analysis of the formulation to ensure compliance with regulations.
Essential Tips
Here are some key tips for achieving FSSAI food compliance:
- Ensure accurate listing of all ingredients and additives.
- Adhere to the quality standards set by FSSAI for the product.
- Ensure compliance with the latest Food Safety and Standards (Labelling and Display) Regulations, 2020, for the product label.
- Consider reformulating the product, if necessary, to eliminate non-compliant ingredients or to enhance its value and potentially bypass the need for NSF approval.
- Step 5: After the review, the State Licensing Authority will grant the manufacturer permission and the manufacturing license in Form MD-5
Form Names: NA
FSSAI Advertisement Claim Approval For Food Supplements
FSSAI advertisement claim approval ensure that advertisements align with food labels, avoid deception, and provide clear information to consumers. Misleading claims can result in penalties, emphasizing the importance of claim approval for food businesses.
Regulatory Body Requirement
The Food Safety and Standards Authority of India (FSSAI) oversees Claims and Advertisements for Food Supplements and Nutraceuticals in India. Manufacturers of nutritional and health products must obtain prior approval before making any health or nutritional claims, or claims related to the reduction of disease risk, to ensure consumers are not misled by advertisements. The FSS (Claims and Advertisements) Regulations, 2018, aim to promote fairness in the claims and advertisements of food products.
How to Apply
To obtain FSSAI Advertisement & Claims Approval, the applicant should follow the below procedure:- Step 1: The applicant must check for the feasibility of claim approval associated with the ingredient/nutrient/product.
- Step 2: The applicant must prepare the Claim Support Dossier (CSD) in prescribed formats along with data suggested by FSSAI.
- Step 3: As a pre-requisite charge, the applicant must prepare a demand draft of Rs. 50,000.
- Step 4: The applicant must submit the duly filled application form, CSD, and demand draft to the FSSAI Office.
- Step 5: The maximum claim per application is three claims.
- Step 6: Finally, the applicant must wait until the application scrutiny/query/approval/rejection is done.
Form Names: Form B & C
FSSAI License For Food Supplements
The acquisition of an FSSAI license is a pivotal step for Food Business Operators (FBOs) in ensuring the safety, quality, and integrity of the food products they offer to consumers. This license serves as a hallmark of compliance with stringent food safety standards and regulations mandated by the FSSAI, thereby affirming that the food is safe for consumption and does not pose any adverse health consequences.
Regulatory Body Requirement
The Food Safety and Standards Authority of India (FSSAI) oversees food-related licensing in India. Manufacturers of functional food supplements are required to possess a valid FSSAI License. This license ensures that Food Business Operators (FBOs) are registered with FSSAI, facilitating compliance with regulations such as food safety audits, annual returns, product testing, and recalls. It serves as a mechanism for FBOs to ensure adherence to FSSAI licensing guidelines.
How to Apply
To obtain an FSSAI License, applicants must follow these steps:- Step 1: Visit the FoSCoS website for food licensing and registration.
- Step 2: Register and log in on FoSCoS with a username, password, and captcha.
- Step 3: Select "Apply for New License/Registration" from the dashboard.
- Step 4: Provide necessary details regarding premises and types of businesses (KoBs).
- Step 5: Complete Form B and attach required documents like Aadhaar Card, Form IX, and proof of premises.
- Step 6: Preview and submit the application, then proceed with payment.
- Step 7: Upon payment completion, receive a receipt with a 17-digit reference number for future use.
- Step 8: The Food Authority grants the license after scrutinizing the application and documents.
Form Names: Form I & II
FSSAI Approval Of Non-Specified Ingredients For Food Supplements
The food supplement industry is rapidly evolving with new innovations and ingredients. Global trade has made it easier to access novel products, but these may not be regulated by FSSAI. Therefore, it's crucial to register and obtain FSSAI approval for these products before manufacturing them in India for commercial use, under the category of Non-Specified Foods.
Regulatory Body Requirement
The Food Safety and Standards Authority of India (FSSAI) is the governing body responsible for approving all food products in India. This service aims to grant approvals for Non-Specified Ingredients/Products - for Food Supplements to manufacturers prior to product launch. The approval process typically spans from six to twelve months.
How to Apply
The applicant must follow these steps:
- Step 1: Prepare the dossier in the specified format, including necessary scientific information and study details.
- Step 2: Fill out Form-I, upload required documents, and pay the online fee of 50,000 rupees, GST inclusive.
- Step 3: The expert committee appointed by the Food Authority will conduct an initial review of the application and provided data. Any deficiencies will be notified to the applicant within 45 days of application receipt.
- Step 4: The FBO should address any required information within 30 days of receiving the notification.
- Step 5: After further evaluation and in accordance with FORM-II, the Food Authority may approve or reject the application.
- Step 6: Upon approval, the FBO must conduct and submit post-market surveillance data on relevant safety and efficacy parameters within one year of product placement.
- Step 7: If the application is rejected, the FBO may appeal to the CEO within thirty days of receiving the rejection notice.
- Step 8: In case of rejection by the appellate Authority, the FBO may file a review petition before the Chairperson of FSSAI within thirty days of the appellate order issuance.
- Step 9: The decision of the Chairperson shall be final.
Frequently Asked Questions
What types of health claims can be made for functional foods?

What kind of stability testing is necessary for functional food products?
Manufacturers are required to conduct shelf-life studies to evaluate the product's stability over time. These studies assess factors such as temperature, humidity, and light exposure to ensure that the product maintains its safety and efficacy throughout its intended shelf life.

How can manufacturers stay informed about emerging trends and research in functional foods?
Regularly monitor scientific literature, attend conferences, and collaborate with experts in the field. Staying up-to-date ensures that manufacturers can adapt their products to meet consumer demands and scientific advancements.

What labeling standards must be followed for functional food supplements?

How can manufacturers guarantee the quality and safety of their functional food supplements?
Manufacturers can ensure quality and safety by adhering to good manufacturing practices (GMP) and conducting thorough testing for purity, potency, and safety.

Contact us
Please feel free to talk to us if you have any questions. We endeavour to answer within 24 hours.
