Non Specified Product / Ingredient Approval in India - Form I & Form II

Avail your FSSAI non-specified food products & ingredients approval in form I and form II. Our team has significant experience in assisting a range of Food industry players to obtain the license for non-specified FSSAI product approval / non-specified food ingredients.
FSSAI Non Specified Food Products / Ingredients Approval – Overview
- Collect the list of necessary documents and their receipts.
- GAP analysis performed by the CE team.A dossier that is compiled as per Form-I.
- The applicant must submit a demand draft of INR 50,000 along with Form-I, which is in favor of the Senior Accounts Officer or FSSAI.
- After submission, the FSSAI will go through the application and respond in cases of queries.
- The applicant must respond to these queries within 15-30 days (or within the stipulated time).
- After addressing the queries, the FSSAI will discuss the application with the scientific panel.
- The FSSAI approves grants non-specified food/ingredient approval if the scientific panel finds the detains convincing or vice versa.
Who Can Apply?
Food Business Operator who wants to manufacture / innovate /import food products which are not specified by FSSAI (and are other than proprietary foods), the Non Specified Product / Ingredient approval will have to be obtained from FSSAI. Precisely, the products with the following categories can apply for Non-specified food approval:
- Novel food or novel food Ingredients or processed with the use of novel technology
- New additives
- New processing aids including enzymes
- Articles of food and food ingredients consisting of or isolated from microorganisms, bacteria, yeast, fungi or algae.
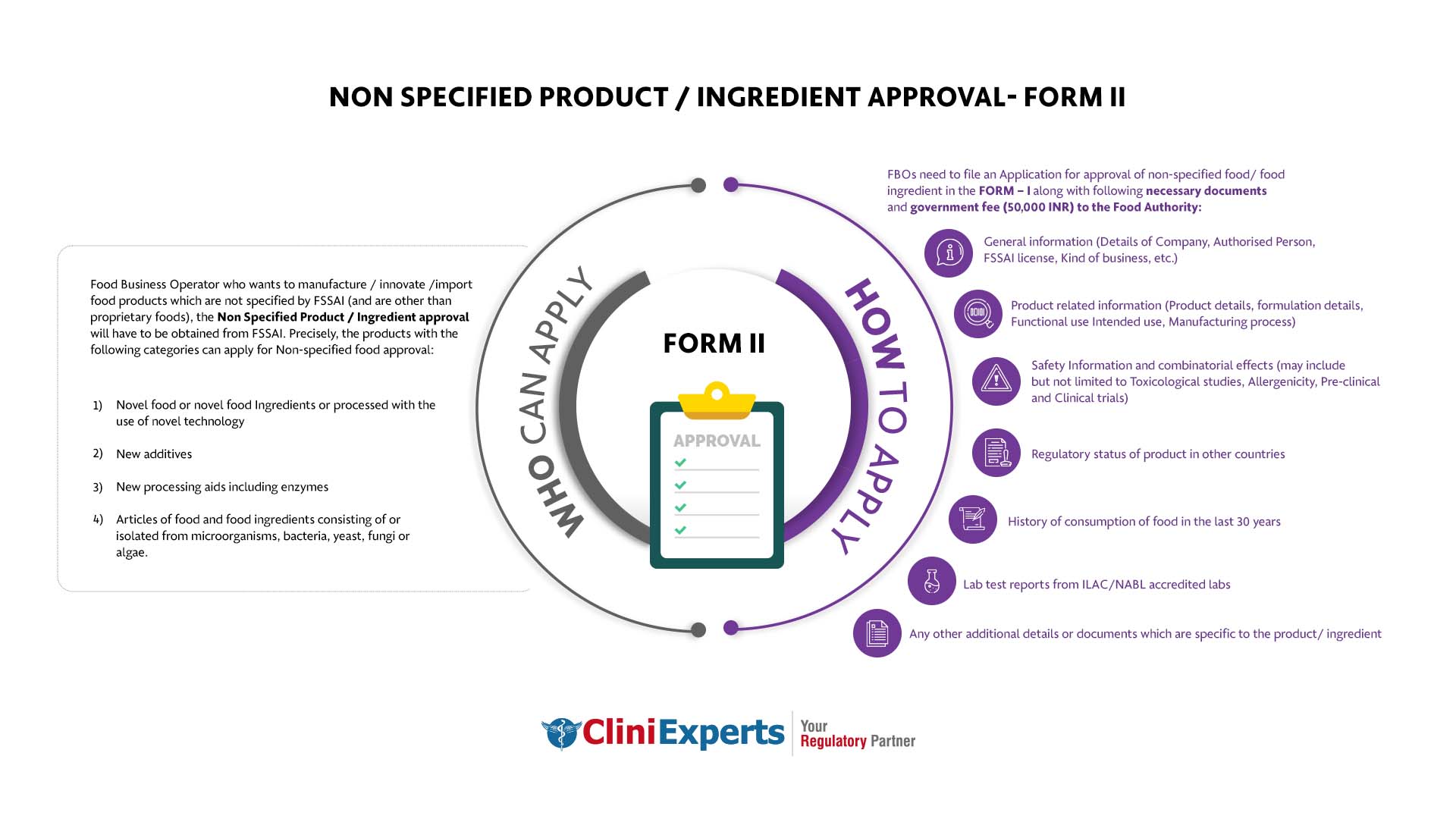
How To Apply?
The Applicant must follow the following process:
-

FBOs need to file an Application for approval of non-specified food/ food ingredient in the FORM – I along with following necessary documents and government fee (50,000 INR) to the Food Authority.
-

General information (Details of Company, Authorised Person, FSSAI license, Kind of business, etc.)
-

Product related information (Product details, formulation details, Functional use Intended use, Manufacturing process)
-

Safety Information and combinatorial effects (may include but not limited to Toxicological studies, Allergenicity, Pre-clinical and Clinical trials)
-

Regulatory status of product in other countries.
-

History of consumption of food in the last 30 years.
-

Lab test reports from ILAC/NABL accredited labs.
-

Any other additional details or documents which are specific to the product/ ingredient.
-

The Food Authority may either grant approval or reject the application, as per FORM-II, on the basis of the safety assessment of the article of food.

Validity
This license is valid for a lifetime.

Fee Involved
To obtain non-specified food approval by FSSAI, the applicant is required to pay INR 50,000 per application.Important Documents

To seek non-specified food approval for initiating business activity in India, the applicant must submit the following documents:
- An FSSAI license of the food product.
- Demand draft of Rs. 50,000, which must be payable to the Senior Accounts Officer of the FSSAI.
- The source, detailed composition, functional use, intended use, and safety information of the ingredient or the food product.
- Agreement of relation between the applicant, manufacturer, and other partners involved.
- The accreditation and certificate of analysis (COA) were obtained from the National Accreditation Board for Testing and Calibration Laboratories (NABL)/International Laboratory Accreditation Cooperation (ILAC).
- The details of the manufacturing process of the food product.
- The regulatory status of the ingredient or food product worldwide.
- Details of any new technology.
- The history of quantity and duration (years) of consumption of food product or ingredient.
- References such as a scientific journal.
Timeline to get
Form II
from FSSAI
9 to 12
MONTHSEssential Tips
To avoid any queries or delays in the approval, the applicant looking for permission for non-specified food approval must ensure these essentials are followed:
- The applicant must select the food product or ingredient category carefully to reduce the chances of rejection.
- The safety details of the food product or ingredients must be available. If the safety data is not sufficient, the applicant must submit human clinical trials data for safety.
- The efficacy, functional use, and dosage of the product must match as per the claims.
- An incorrect selection of food products or ingredients can raise the chances of queries (if the incorrect selection occurs frequently).
- Incorrect interpretation of the regulatory status of the food product or ingredient can raise queries.
- The applicant must not submit an in-house COA. The only COA approved by NABL/ILAC are mandatory.
Expert Advise
The applicant must ensure that they include only the nutritional benefits of the food product or ingredient. Drug-like application of the food product or ingredient leads to rejection.
The applicant should only submit non-GM food products to the FSSAI.
If the product contains more than one ingredient, the applicant must ensure that the combinatorial effects of these ingredients are safe and efficacious.
Frequently Asked Questions
What types of food products or ingredients are covered under these regulations?
The following food products or ingredients are covered under these regulations:
- Novel food or novel food ingredients.
- New additives or new processing aids (including enzymes).
- Articles/papers of food products or ingredients that contain or are isolated from microorganisms, bacteria, yeast, fungi, or algae.
- Other non-specified food.

What is a novel food or food ingredient as per the FSSAI?
A novel food or food ingredient is the one that itself or any ingredient used in it does not have any history of human consumption. This also includes the food product or ingredients derived from any innovative engineering process, which changes the composition or structure of food, thereby altering the nutritional content, metabolism, or the levels of unwanted substances.

What is a new additive as per the FSSAI?
A new additive can be defined as an ingredient that has not been included in any specific food category per the Food Safety and Standards Regulations (FSSR). The products listed under this category also include the additive assessed for safety by the Joint FAO/WHO Expert Committee on Food Additives (JECFA). The food additive is included in Codex and approved by other regulatory authorities.

What is a processing aid as per the FSSAI?
A processing aid is not included in FSSR, but Codex and other regulatory bodies are categorized under new processing aid.

What does the COA should include?
A COA should include parameters relevant to the food product or ingredient like:
- Physical parameters
- Nutritional details
- Active ingredient
- Heavy metals
- Residues
- Microbiological parameters
- Pesticide residues
- Naturally occurring toxicants
- Validated test method
- Other tests method
- References, wherever applicable.

