Registration of Foreign Food Manufacturing Facilities in India - Form 16 & Form 17

CliniExperts provides 360-degree regulatory solutions. We have over a decade of experience working with industry regulators. With our deep industry connections and up-to-date team, be assured that your products will be granted the required licenses.
Registration of foreign manufacturer facility (ReFOM) – Overview
- The Food Authority reviews the application and may request additional documents if necessary.
- In response to a complete application, the Authority may inspect or issue a registration/rejection.
- Foreign food manufacturing facilities must register if they comply with the Food Safety and Standards Act. Typically, the license processing is completed within 60-120 days.

Who Can Apply?
A foreign food manufacturing facility that wants to export high-risk food categories to India may apply.
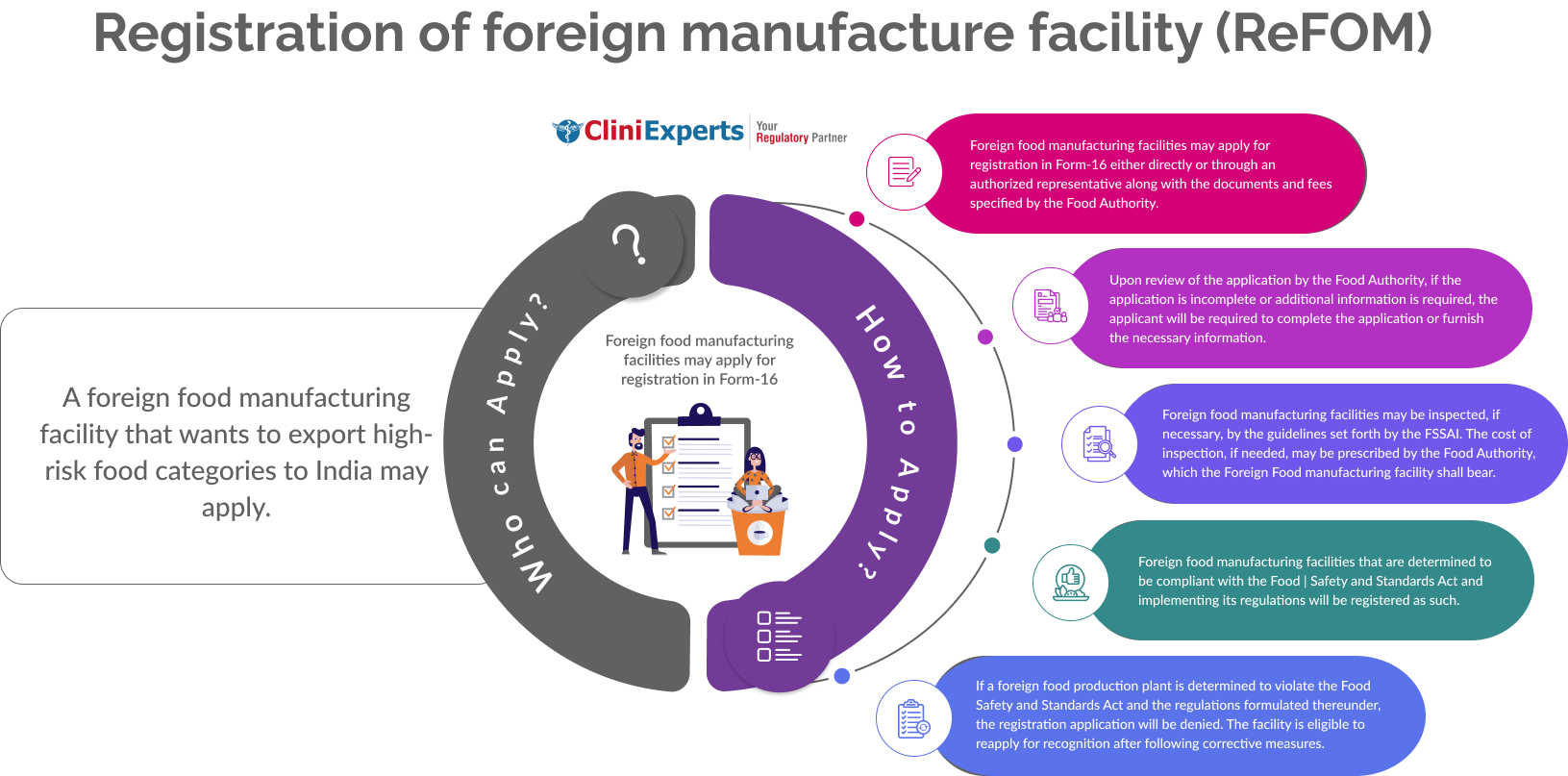
How To Apply?
The Applicant must follow the following process:
-

Foreign food manufacturing facilities can register using Form-16 or through an authorized agent. They must submit specified documents and fees as required by the Food Authority.
-

Upon review of the application, if its incomplete or some information is required, the applicant must furnish all the details.
-

Foreign food manufacturing facilities may be inspected, if necessary, by the guidelines set forth by the FSSAI.
-

The cost of inspection may be prescribed by the Food Authority, which the Foreign Food manufacturing facility shall bear.
-

If a foreign food production plant is determined to violate the Food Safety and Standards Act, the application will be denied. The facility is eligible to reapply for recognition after following the corrective measures.

Validity
The license is valid for 2 years from the date of issuing the registration.

Fee Involved
The fee has not been specified by the Food Authority.Important Documents

Several key documents needed are as follows:
- Form-16
- Detailed composition of products
- Consent of the owner of Foreign Food manufacturing facility for inspection of the facility by the FSSAI officials
- License/RC or similar document issued by the concerned country’s government authority
- Authorization in favor of an authorized representative (if applied through an authorized representative).
Timeline to get
Form 17
from FSSAI
60 to 120
DaysEssential Tips
One should remember the following essential things when submitting the license application:
- The product's Harmonized System (HS) Code is required.
- If the authority requests additional information, it must be submitted within the allotted time frame. Otherwise, the application will be denied.
- All documents should be on the business's letterhead and should be signed and stamped.
- The obstacles or issues that are most likely to arise when filling out or applying are:
- Insufficient records or data
- Any information or document supplied is inaccurate and not true to nature.
- If the inspection report is not as per the Food Safety and Standards Act, regulations, and guidelines.
Expert Advise
The documentation provided should be as specified by the food authority and needs to be furnished within the given timeline.
The manufacturing facility should be up to the mark, in case authority orders an inspection.
Composition of the product should be true to its nature.
Related Services
FSSAI Food License Registration (Form C)
Importer/Manufacturer | Regulatory Body: FSSAI
Grab your FosCos FSSAI License / Registration in India (Form A, B, and C). CliniExperts team works as FSSAI consultants and has significant experience in assisting a range of Food industry players obtain the FSSAI Food License Registration that is appropriate to the size and nature of their business.
Frequently Asked Questions
What food categories are eligible for registration?
The following food categories are intended to be exported to India:
- Milk and milk products
- Meat and meat products including poultry, fish, and their products
- Egg powder
- Infant foods
- Nutraceuticals and other food supplements

Is the registration issued for the head office or the premises?
The registration will be issued for the premise address.

Is the registration renewable or do we need to apply for the registration again after the expiry?
Yes, the registration can be renewed; there is no need for a new application. Renewal of registration of Foreign Food manufacturing facility shall be made in Form 16, not later than thirty days before the expiry date indicated in the registration.

Does the foreign manufacturing facility require an FSSAI License (Form-C)?

Is it possible for the facility to be inspected after the registration is issued?
Yes, foreign Food manufacturing facilities may also be inspected after the issuance of registration, as deemed necessary. Provided that no inspection shall be required in case of such categories of food that are covered under the mandatory Bureau of Indian Standards Certification Mark Scheme and where the Bureau of Indian Standards scheme of inspection includes the requirements specified under Schedule 4 of the Food Safety and Standards (Licensing and Registration of Food Businesses) Regulations, 2011.

