Clarification Letter / No Objection Certificate for Medical Devices in India - NOC

Unclear about the regulatory status of Medical Devices in India. Let CliniExperts’ professionals assist you for getting a Clarification Letter / No Objection Certificate (NOC) for Medical Devices from Central Drugs Standard Control Organisation.
Clarification Letter For Medical Devices from CDSCO (NOC) – Overview
The Central Drugs Standard Control Organization (CDSCO) has listed new regulations to obtain clarification on the regulatory status of the Medical Devices. To get this clarification, the importer or manufacturer must submit a no-objection certificate (NOC). The applicant should submit a hard copy of the NOC to the Medical Device division in CDSCO.
Who Can Apply?
Any Importer and local manufacturer can apply for Clarification NOC from CDSCO
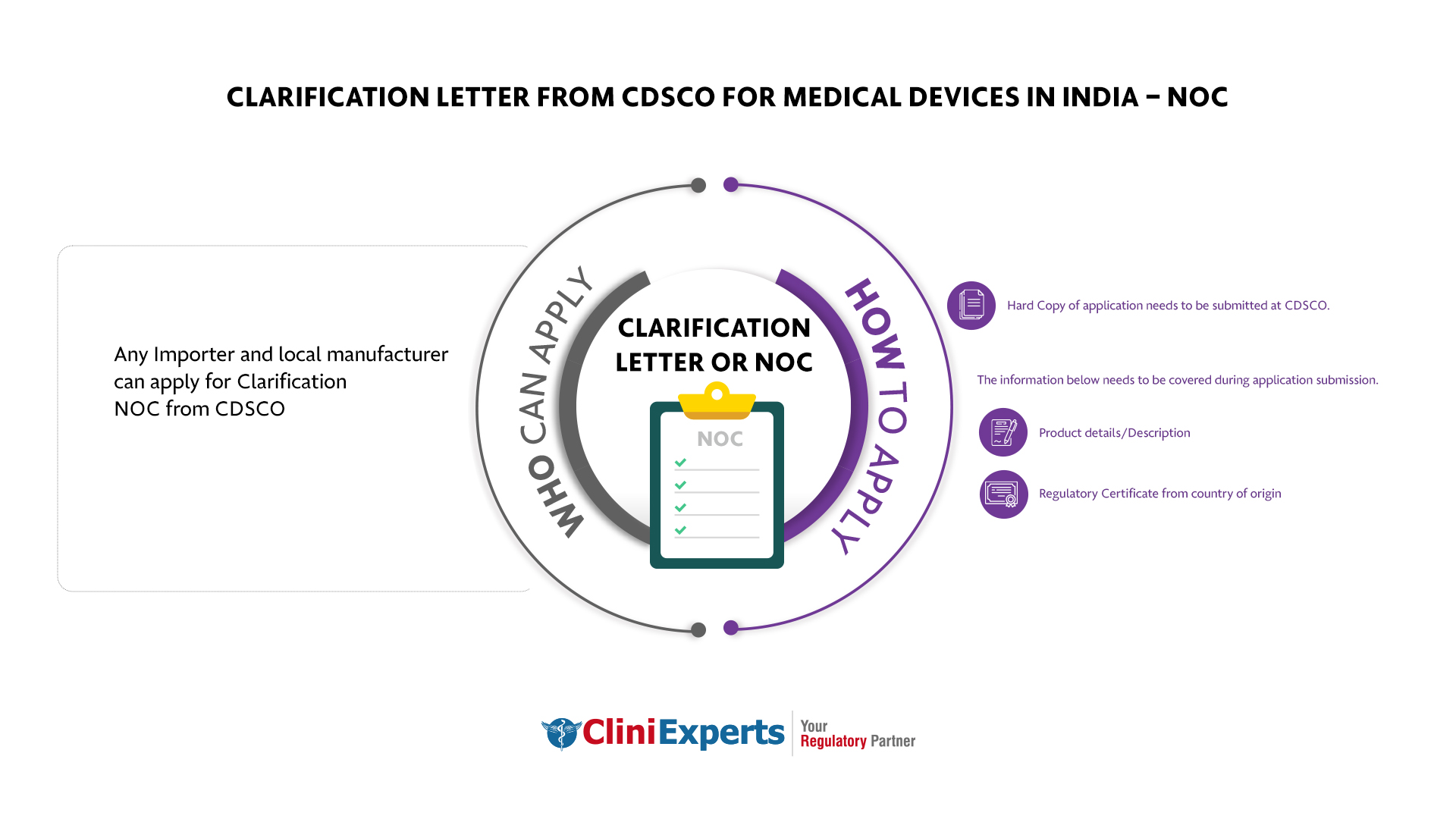
How To Apply?
The Applicant must follow the following process:
-

Product details/Description
-

Regulatory Certificate from country of origin

Validity
The validity of this is not specified.

Fee Involved
No fees will be required to obtain clarity regarding the regulatory status of the productImportant Documents

To submit a NOC to the Medical Device division in CDSCO, the importer or manufacturer must also submit the following documents:
- The details or description of the Medical Device.
- A regulatory certificate from the country of origin of the Medical Device.
Timeline to get
NOC
from Central Drugs Standard Control Organisation
Not specified
Essential Tips
The importer or manufacturer must ensure that they have essential documents containing details of the Medical Device and the regulatory certificate from the country of origin of the Medical Device. As the timeline is not specified by the CDSCO, the application can take more than 30 days to process.
- Product details/ Description
- Regulatory Certificate from country of origin
Expert Advise
The importer or manufacturer should ensure that they have listed the exact intended use of the Medical Device as claimed in the instructions for use.
Related Services
Permission to import or manufacture new medical device in India
Importer | Regulatory Body: CDSCO
Get experts assistance to avail Permission to import or manufacture medical device which does not have its predicate device in India -As per MDR 2017
Non-Notified Medical Devices Registration/ Approval in India
Importer | Regulatory Body: CDSCO
CliniExperts acts as an authorized representative to help you in getting the permission to import non-notified medical devices in India. Register your Non-notified Medical Devices in India with CliniExperts' professional assistance.
Import License for Medical Devices in India
Importer | Regulatory Body: CDSCO
Meet all your Regulatory Compliance needs. CliniExperts' professionals help you plan and streamline regulatory approval processes.
Medical Device ISO 13485 Certification Assistance in India
Importer | Regulatory Body: CDSCO
Medical Device ISO 13485 Certification is a comprehensive framework for manufacturers, importers, and distributors that maintain the quality of medical devices' regulatory compliance. Contact CliniExperts to get Medical Devices ISO 13485 Certification Assistance and stay ahead of the competition by assuring utmost safety and efficacy.
Frequently Asked Questions
What does clarification NOC mean?
A clarification NOC is a no-objection certificate issued by the CDSCO, which reflects the regulatory status of the Medical Device in India.

Who are eligible for applying for clarification NOC?
Both importers and manufacturers can apply for clarification NOC

