EPR Authorization Certificate from CPCB in India

Elevate your environmental compliance with CliniExperts! Obtain your EPR Authorization Certificate from CPCB hassle-free. Rely on our industry network and updated team for swift approvals. Streamline your regulatory journey today! Contact us for unparalleled support.
EPR Authorization Certificate from CPCB – Overview
Who Can Apply?
All the producers, importers, and brand owners of electronic or electrical equipment, plastic packaging, or batteries are eligible to apply.
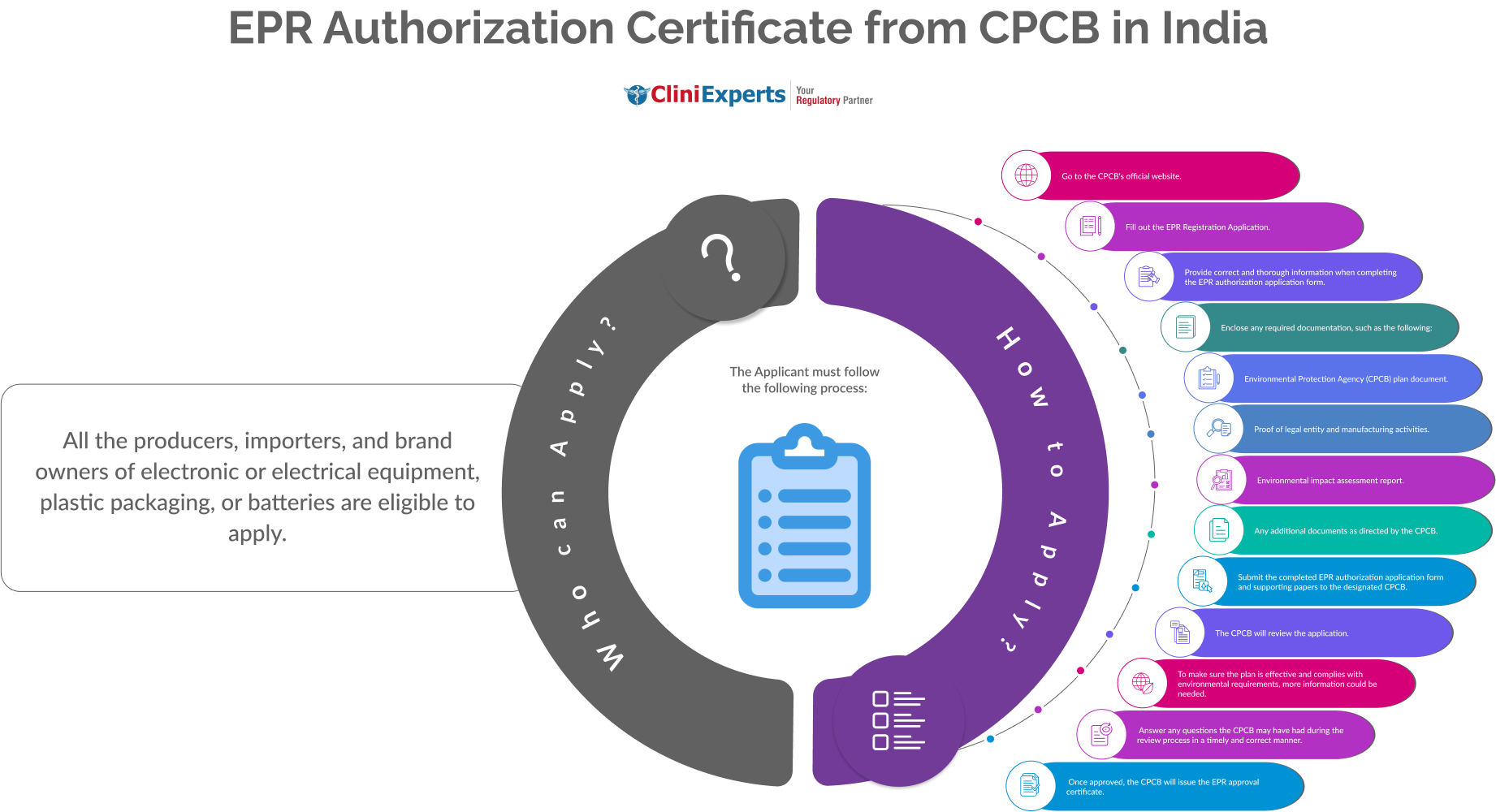
How To Apply?
The Applicant must follow the following process:
-

Go to the CPCB's official website.
-

Fill out the EPR Registration Application.
-

Provide correct and thorough information when completing the EPR authorization application form.
-

Enclose any required documentation, such as Environmental Protection Agency plan document, proof of legal entity and manufacturing activities, environmental impact assessment report, Any additional documents as directed by the CPCB.
-

Submit the completed EPR authorization application form and supporting papers to the designated CPCB.
-

The CPCB will review the application.
-

To make sure the plan is effective and complies with environmental requirements, more information could be needed.
-

Answer any questions the CPCB may have had during the review process in a timely and correct manner.
-

Once approved, the CPCB will issue the EPR approval certificate.

Validity
The license validity is different for the different categories of the waste:
- Plastic waste- Lifetime
- Electronic waste- 5 years
- Battery waste- 5 years

Fee Involved
The government charges for the application process vary from case to case.
Important Documents

- 1. Details and company
- PAN card and GST certificate
- CIN of the company
- IEC certificate of the importer
- Partnership deed in case of a partnership firm
- 2. Details of authorized person
- Aadhar details
- PAN of the authorized person
- Scanned copy of signatures of authorized persons
- 3. Other important documents
- DIC registration (if the unit registered is with DIC)
- Covering letter
- Any other relevant document regarding any information which the unit wishes to provide
Timeline to get from CPCB
2-3
MonthsEssential Tips
The primary considerations that need to be made while preparing and submitting this loan license application are:
- Recognize Applicability: Find out if the EPR regulations apply to the operations of your company.
- Adherence to CPCB Regulations: Verify adherence to the particular directives and regulations concerning EPR.
- Documentation: As needed by the CPCB, prepare an complete set of documentation.
The obstacles or issues that are most likely to arise when filling out or applying are:
- Absence of Follow-Up
- Regulatory Changes
- Lack of Expert Guidance
Expert Advise
According to our experts, the applicants (producers, importers, brand owners) should look out for the following license application procedure:
The company's details must be correct.
All the supporting documents must be current as of the application submission date.
Precise information about the waste generated must be provided during the application submission procedure.
Related Services
Test license to import In Vitro Diagnostics (Form MD 16, 17)
Importers | Regulatory Body: CDSCO
Do you need permission to import in-vitro diagnostic kits to India to demonstrate its performance? CliniExperts’ professionals have the expertise and assist you in securing in-vitro diagnostic kits test license for importer.
Permission to conduct Clinical Performance Evaluation (Form MD 24, 25)
Manufacturer / Importer | Regulatory Body: CDSCO
CliniExperts is backed by a well-informed and experienced team who guides through all the documentation and paperwork required for the regulatory processes.
Permission to Manufacture Class C & D In- Vitro Diagnostics (Form MD 7, 9)
Manufacturer | Regulatory Body: CDSCO
At CliniExperts, our team of experts is thoroughly updated with the latest requirements of the various authorities, enabling complete and meticulous documentation, timely application and quick approvals/licenses.
Permission to Manufacture Class A & B In Vitro Diagnostics (Form MD 3, 5)
Manufacturer | Regulatory Body: CDSCO
The application for manufacturing, sale or distribution of any medical device includes a tedious list of processes and forms CliniExperts team consists of experts working in the same domain with practical experience and knowledge of the governing rules.
Frequently Asked Questions
What constitutes the CPCB, and what are its principal goals?

How does the CPCB enforce environmental standards, and what are the penalties for breaking its guidelines?
If CPCB guidelines are not followed, there could be fines, penalties, or legal action decided on case basis.

What is Extended Producer Responsibility (EPR)?
Extended producer responsibility (EPR) stands for ecologically sound product management, which is the producer's obligation across the product's life cycle.

Which are the plastic packaging categories covered under EPR?
- Category I: Rigid plastic packaging.
- Category II: Flexible plastic packaging like sachets, pouches, carry bags, plastic sachets, and sheets; multilayer packaging (more than one layer with various types of plastic); and similar packaging.
- Category III: Plastic packaging with multiple layers (at least one layer of plastic and one layer of a material other than plastic).
- Category IV: Compostable plastic carry bags and sheets or similar materials used for packaging.

Who remains exempted from the e-waste (M) rules, 2016?
The e-waste (M) rules, 2016 shall not apply to:
- Used lead acid batteries in accordance with the guidelines established by the act's batteries (Management and Handling) Rules, 2001.
- Micro-enterprises as defined by the Micro, Small and Medium Enterprises Development Act, 2006 (27 of 2006).
- Radioactive waste as defined by the Atomic Energy Act of 1962 (33 of 1962) and its implementing regulations.

