Complete Procedure to Meet the CDSCO License Mandate of Class C & D Non-Notified Medical Devices
Medical Device
October 25, 2023
Overview of the CDSCO License Mandate The Central Drugs Standard Control Organisation (CDSCO) is the regulatory body that manages medical device registration in India under the Ministry of Health & Family Welfare. The main aim of CDSCO is to ensure that medical devices are...
Read More


Blog
January 2, 2013
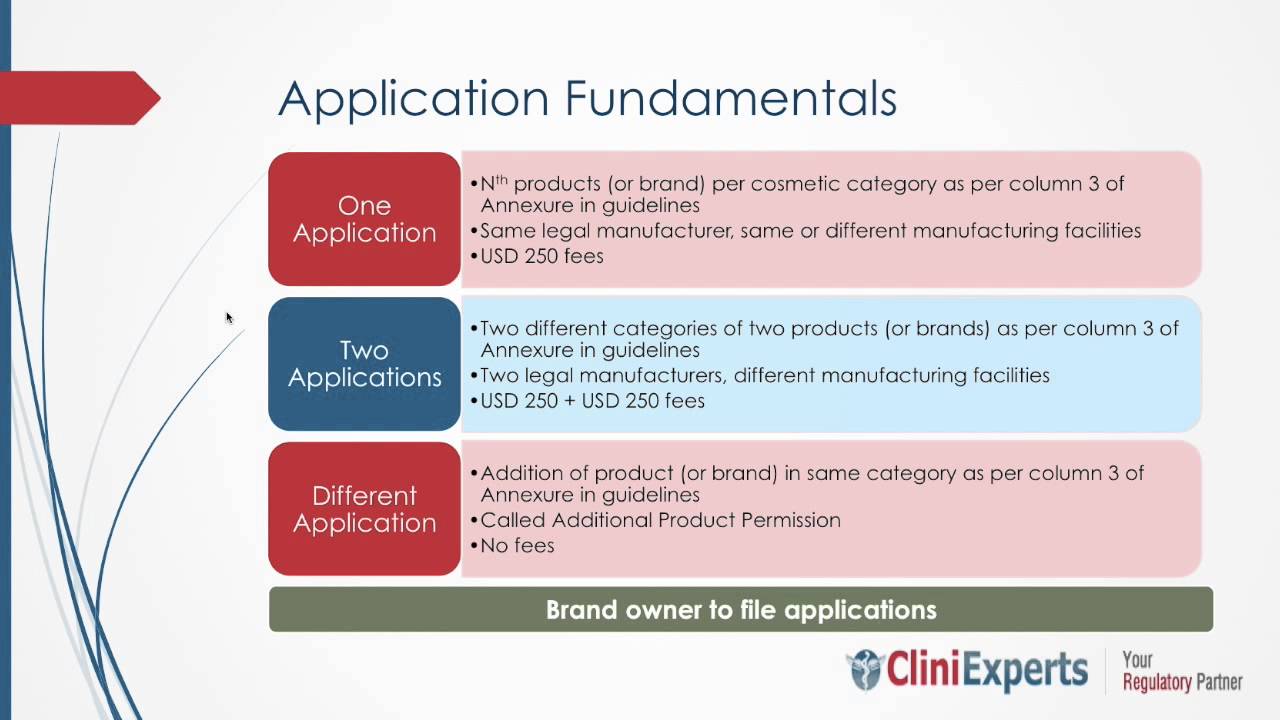
Guidelines on Registration of Import of Cosmetics (AS ON 2.1.2013) A: PURPOSE: 1. The Government of India has issued a Gazette Notification G.S.R 426(E) dated 19 th May 2010 for amending the...

Blog
November 26, 2012
The Drug Controller General of India (DCGI) has planned to meet senior officials from all the zonal offices across the country in the coming week to assess and examine the regulatory mechanism...

Blog
November 21, 2012
(Reuters) – European health regulators on Tuesday approved an eagerly anticipated blood thinner developed by Bristol-Myers Squibb Co and Pfizer Inc for preventing strokes and blood clots in patients with an irregular...

Blog
November 6, 2012
(CNN) – When you’re a patient, you trust you’re in good hands, but even the best doctor or nurse can make a mistake on you or someone you love. “Mistakes are happening every...

Blog
August 7, 2012
Ginger, the common spice and ancient Asian remedy, could have the power to help manage the high levels of blood sugar which create complications for long-term diabetic patients, a University of Sydney study reports....

Blog
July 31, 2012
While the number of centers is growing, payer pressure and new technologies have forced them to adapt or face financial struggle. How physicians responded to financial problems in one corner of the...

Blog
July 31, 2012
Physicians applaud the latest step in reducing new HIV cases, but insist that high-risk individuals who take the medication still need to practice safe sex. After decades of health professionals trying to...

Blog
July 28, 2012
Under rule 122 DD Registration of Ethics committee (EC) – No ethics committee shall review and accord its approval to a clinical trial protocol without prior registration of the committee with the...
End-to-End Regulatory Solutions for Domestic and International Markets
ENQUIRE NOW
KNOW MORE, LEARN MORE, ENGAGE MORE.
“Video-Only” Resource For Ease Of Understanding