Table of Contents
Summary of New Drug, New FDC and SND as per Drug and Cosmetic Act

What is a New Drug as per Drug and Cosmetic Rules (D&C)?
As per Rule 122 E of the Drug and Cosmetic Rules 1945, a New Drug can be – [1]
- A drug, including bulk drug substance which has not been used in the country and has not been recognized as effective and safe by the licensing authority for the proposed claims.
- An approved drug with modified or new claims like –
- New Indications
- New Dosage
- New Dosage Form (including sustained release dosage form)
- New Route of Administration
- A fixed dose combination of two or more drugs, individually approved earlier for certain claims, which are now proposed to be-
a) Combined for the first time in a fixed ratio OR
b) If the ratio of ingredients in an already marketed combination is proposed to be changed, with certain claims, which can be-
(i) New Indications
(ii) New Dosage
(iii) New Dosage Form (including sustained release dosage form.
(iv) New Route of Administration
4. All vaccines shall be new drugs unless certified otherwise by the Licensing Authority.
5. A new drug shall continue to be considered as new drug for a period of four years from the date of its first approval or its inclusion in the Indian Pharmacopoeia, whichever is earlier. [2]
What Is A Fixed Dose Combination?
Fixed Dose Combinations (FDC’s) refer to products containing two or more active ingredients used for a particular indication(s). As per Rule 122-E of Drugs & Cosmetics Rule, a fixed dose combination of two or more drugs, individually approved earlier for certain claims, which are now proposed to be combined for the first time in a fixed ratio, or if the ratio of ingredients in an already marketed combination is proposed to be changed, with certain claims, viz. indications, dosage, dosage form (including sustained release dosage form) and route of administration are considered new drugs. Further, a new drug shall continue to be considered as new drug for a period of four years from the date of its first approval or its inclusion in the Indian Pharmacopoeia, whichever is earlier. [1]
FDC can be classified into 5 categories. Following are the classification of FDC [3]:
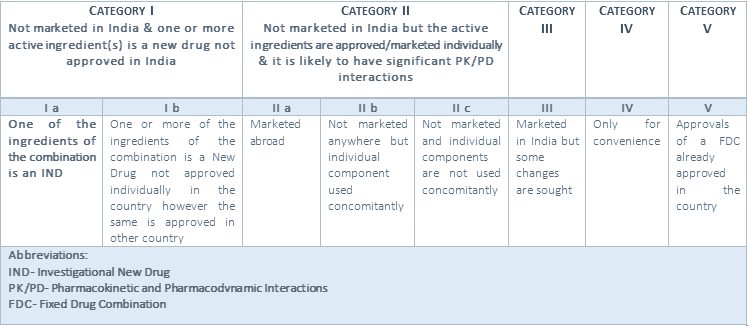
FDC Categories and Classification
What Is A Subsequent New Drug Application?
A Subsequent New Drug application is an application for approval of an already approved new drug by the Central Drugs Standard Control Organization (CDSCO). Subsequent new drug application can be made for the following cases:
- Bulk Drug already approved in the country (approved within 4 years).
- New drug (Formulation) already approved in the country.
- A drug already approved and proposed to be marketed with new indication.
- A drug already approved and proposed to be marketed as a ‘New Dosage Form / New Route of Administration’.
- A drug already approved and proposed to be marketed as a ‘Modified release dosage form’.
- A drug already approved and proposed to be marketed with Additional Strength.
All the applications for approval of New Drug, Fixed Dose Combination and Subsequent New Drug are made under Form 44 (Application for grant of permission to import or manufacture a New Drug or to undertake clinical trial).
References
- Directorate General of Health Services. The Drugs and Cosmetics Act and Rules. D&C Rule’s 1945- 122E:2005. Delhi: D&C; 2005. p. 134.
- Central Drugs Standard Control Organization (CDSCO). New Drug Checklist. Available from: http://www.cdsco.nic.in/forms/list.aspx?lid=2053&Id=3 [Accessed 19th February 2015].
- Central Drugs Standard Control Organization (CDSCO). FDC Checklist. Available from: http://www.cdsco.nic.in/forms/list.aspx?lid=2053&Id=3 [Accessed 19th February 2015].
Recent Posts
Organic Food Labelling In India| Certification, and Import of Organic Food in India

This Article is All About Organic Food Labelling In India and Certification, and Import of Organic Food in India. Explained in Detail About What is Organic Food labelling? Summary Short Description Wi..
Cosmetic Label Compliance India : A Guide to Compliance

Introduction Looking for Cosmetic Label Compliance India? Are you a cosmetic manufacturer or importer navigating the complex world of Indian regulations? Ensuring your product labels comply with the l..
Clinical Investigation Approvals: An Overview of Forms MD-22 and MD-23

Summary Short Description Strict regulatory protocols govern clinical investigations for medical devices. Central to this process are forms MD-22 and MD-23. Form MD-22 is an application to Central Lic..
HAVE A QUERY?
REACH US!Office
New Delhi
Unit No. 324 & 325, City Centre Mall, Plot No. 5, Sector 12, Dwarka, India - 110075
+917672005050
Bengaluru
RMZ Galleria, 1st floor, Ambedkar Colony, Yelahanka, Bengaluru, Karnataka, India – 560064
Call us on
Sales: +91 7672005050
Reception: +91-11-45214546
Timings
9 am to 6 pm (Monday to Friday)


