Table of Contents
The Future of SaMD: Emerging Trends and Innovations

Overview
The future of (SaMD) Software as a Medical Device will see breakthroughs in Artificial Intelligence-based diagnostics, personalised treatments, and remote monitoring.
Emerging trends emphasise real-time data integration, stronger regulatory measures, and enhanced cybersecurity, aiming to transform patient care with greater precision, efficiency, and accessibility.
Short Summary
- When software is designed to treat, diagnose, cure, mitigate, or prevent diseases or other conditions, it is considered a medical device under the Food, Drug, and Cosmetic Act and is referred to as Software as a Medical Device by the Food and Drug Administration and the International Medical Device Regulators Forum.
- Artificial intelligence and machine learning technologies are being used by medical device manufacturers to reinvent their products in order to enhance patient care and support to healthcare providers.
- Careful management is beneficial for the intricate and dynamic processes involved in the creation, implementation, application, and upkeep of artificial intelligence technology throughout the life cycle of medical products.
What Is SaMD?
Software as a Medical Device (SaMD) refers to software designed to be used for one or more medical objectives and accomplishes these objectives independent of any hardware medical device.
The Food and Drugs Administration (FDA) defines a medical purpose as one that aims to treat, diagnose, cure, ameliorate, or prevent disease or other conditions under the Federal Food, Drug, and Cosmetic Act (FD&C Act).
Artificial Intelligence (AI) and Machine Learning (ML) are transforming SaMD by enhancing diagnostics, enabling personalized medicine, and automating tasks. However, integrating these technologies requires the careful management of data privacy, algorithmic biases, and regulatory compliance.
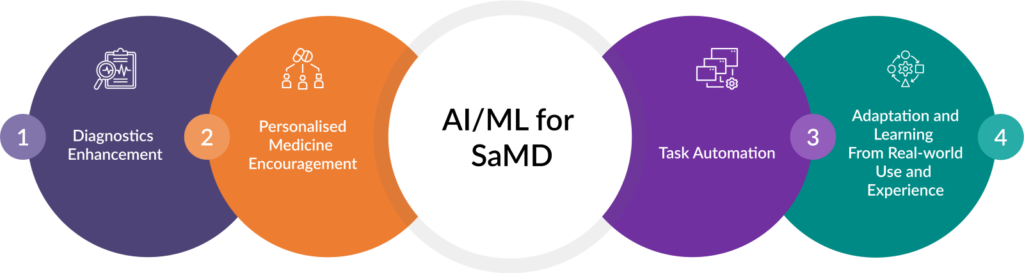
Fig. 1: AI and ML Transforming Devices
AI/ML Software as a Medical Device
The future of AI and ML, especially in the context of SAMD and related fields, is quite promising and multifaceted. AI can use different methods, like ML, to exhibit smart behavior. This includes using data for statistical analysis and expert systems based on if-then rules.
AI and ML can be used to create software that learns from and acts on data. When this software is designed to treat, diagnose, cure, mitigate, or prevent diseases or other conditions, it is considered a medical device under the FD&C Act and is referred to as SaMD by the FDA and the IMDRF.
AI and ML – The Future of SaMD
AI and ML are revolutionising healthcare by utilising large volumes of data to enhance medical devices and improve patient outcomes. AI refers to systems that can make predictions, recommendations, or decisions based on data from real or virtual environments.
ML, a subset of AI, involves training algorithms to improve their performance through experience and data. These technologies enable medical devices to adapt and improve continuously based on real-world usage.
Traditional regulatory pathways, such as premarket clearance (510(k)), De Novo classification, and premarket approval, were not originally designed for the adaptive nature of AI/ML technologies.
However, the FDA is evolving its regulatory framework to address the complexities of AI and ML in medical devices and has issued new guidance documents, including the 2019 discussion paper on AI/ML software modifications, the 2021 “AI/ML SaMD Action Plan,” and subsequent guidelines on change control and transparency.
These initiatives reflect the FDA’s commitment to ensuring the safety and effectiveness of AI-/ML-enabled medical devices while accommodating their dynamic development and use.
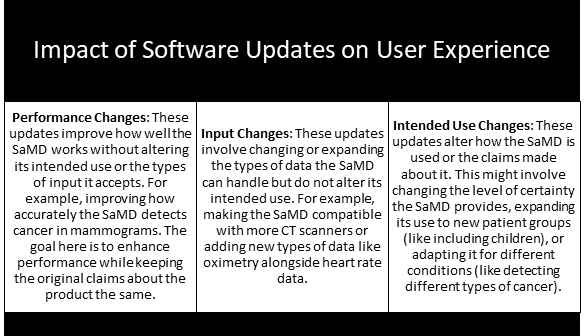
Emerging Trends and Innovations – Types of AI-/ML-based SaMD Modifications
Software updates for AI-/ML-based SaMD can impact users in different ways, including the following:
Conclusion
The future of SaMD promises transformative advancements through AI, personalised medicine, and enhanced regulatory frameworks. As innovation accelerates, SaMD will revolutionise diagnostics and treatment, offering more precise and effective healthcare solutions.
Staying up to date with trends is crucial for leveraging the full potential of SaMD. By drawing new and significant conclusions from the massive amounts of data created every day while providing healthcare, AI and ML technologies have the potential to bring about rapid and significant change in the healthcare industry.
References
- Software as a Medical Device (SaMD) [Internet]. FDA. 2020.
Available from: https://www.fda.gov/medical-devices/digital-health-center-excellence/software-medical-device-samd
- Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) -Discussion Paper and Request for Feedback [Internet].
Available from: https://www.fda.gov/files/medical%20devices/published/US-FDA-Artificial-Intelligence-and-Machine-Learning-Discussion-Paper.pdf
- Software as a Medical Device: Possible Framework for Risk Categorization and Corresponding Considerations | International Medical Device Regulators Forum [Internet]. www.imdrf.org. 2014.
Available from: https://www.imdrf.org/documents/software-medical-device-possible-framework-risk-categorization-and-corresponding-considerations
- Artificial Intelligence and Machine Learning in Software [Internet]. U.S. Food and Drug Administration. 2019.
Available from: https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-software-medical-device
Recent Posts
Organic Food Labelling In India| Certification, and Import of Organic Food in India

This Article is All About Organic Food Labelling In India and Certification, and Import of Organic Food in India. Explained in Detail About What is Organic Food labelling? Summary Short Description Wi..
Cosmetic Label Compliance India : A Guide to Compliance

Introduction Looking for Cosmetic Label Compliance India? Are you a cosmetic manufacturer or importer navigating the complex world of Indian regulations? Ensuring your product labels comply with the l..
Clinical Investigation Approvals: An Overview of Forms MD-22 and MD-23

Summary Short Description Strict regulatory protocols govern clinical investigations for medical devices. Central to this process are forms MD-22 and MD-23. Form MD-22 is an application to Central Lic..
HAVE A QUERY?
REACH US!Office
New Delhi
Unit No. 324 & 325, City Centre Mall, Plot No. 5, Sector 12, Dwarka, India - 110075
+917672005050
Bengaluru
RMZ Galleria, 1st floor, Ambedkar Colony, Yelahanka, Bengaluru, Karnataka, India – 560064
Call us on
Sales: +91 7672005050
Reception: +91-11-45214546
Timings
9 am to 6 pm (Monday to Friday)


