Table of Contents
Clinical Investigation Approvals: An Overview of Forms MD-22 and MD-23

Summary
- As per MDR 2017, “clinical investigation” means the systematic study of an investigational medical device in or on human participants to assess its safety, performance or effectiveness.
- Clinical investigations are crucial for assessing the safety and effectiveness of medical devices, ensuring they comply with regulatory and ethical standards.
- Forms MD-22 and MD-23 are essential for seeking permission and approval to conduct clinical investigations, ensuring regulatory compliance.
- Form MD-22 includes applicant details, device and site information, clinical investigation plan, fee details, and confirmation of legal compliance.
- Form MD-23 grants approval for clinical investigations, detailing device information, investigation sites, approval conditions, and the signature of the Central Licensing Authority.
- Best practices for approvalinclude ensuring Ethics Committee approval, submitting biocompatibility test reports, and providing a comprehensive Clinical Investigation Plan for successful approval.
Short Description
Strict regulatory protocols govern clinical investigations for medical devices. Central to this process are forms MD-22 and MD-23. Form MD-22 is an application to Central Licencing Authority (CLA) for conducting investigation on an investigational medical device whereas MD-23 is the approval of same. These ensure safety, performance and compliance with ethical standards required for medical devices.
Importance of Clinical Investigation Approvals
Clinical investigations are the foundation of medical device development. They help establish whether a device is safe, effective and fit for its intended use. Regulatory bodies, such as Central Licencing Authority (CLA) and Central Drugs Standard Control Organisation (CDSCO), mandate the submission of detailed documentation to ensure that these investigations are conducted ethically and meet required standards.
Understanding the Purpose of Forms MD-22 and MD-23
Forms MD-22 and MD-23 are regulatory documents mandated by competent authorities to monitor and approve clinical investigations involving investigational medical devices. They aim to ensure that the proposed investigations meet the scientific, ethical and legal requirements. Here’s what each form involves:
Form MD-22: An application for permission to conduct a clinical investigation involving an investigational medical device can be submitted using Form MD-22.
Form MD-23: The approval to conduct clinical investigations for the investigational medical device is granted through Form MD-23.
These forms are essential to safeguard public health while enabling the advancement of medical technology.
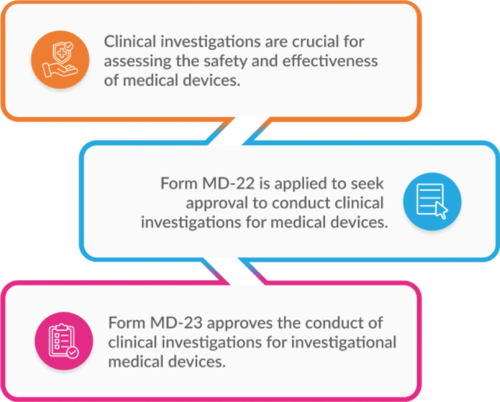
Fig. 1: The Relation Between the Forms MD-22 and MD-23 For the Purpose of Clinical Investigations
Key Components of Form MD-22
Sponsors must submit Form MD-22 to the Central Licensing Authority to seek permission for clinical investigations of investigational medical devices, and the application must include the information specified in the seventh schedule along with the required supporting documents, as outlined in Section 51 of The Medical Devices Rules, 2017.
Below are the key components included in the form:
1. Applicant Details
- Name of the Applicant.
- Nature and Constitution: Specify whether the applicant is a proprietorship, partnership (including LLP), company, society, trust, or other entity.
2. Address Details
- Sponsor’s Address: Includes telephone, mobile, fax, and email details.
- Clinical Investigation Site Address: Complete contact details for the site(s).
- Address for Correspondence: For official communication regarding the application.
3. Investigational Medical Device and Site Information, as per annexures.
- Details of the investigational medical device(s): Annexure including Generic Name, Intended Use, Class of Medical Device, and other technical details.
- Information about the clinical investigation site(s): Annexure including Name and address of the clinical investigation site(s), Ethics Committee Details and Principal Investigator Name.
4. Clinical Investigation Plan
- Plan Number and the corresponding date.
5. Fee Details
- Payment information, including the amount paid and receipt, challan, or transaction ID.
6. Enclosures
- Confirmation of all documents submitted as per the Seventh Schedule of Medical Devices Rules, 2017.
7. Undertaking by the Applicant
- A declaration of compliance with the Drugs and Cosmetics Act, 1940 and the Medical Devices Rules, 2017.
This detailed structure ensures transparency and regulatory compliance, facilitating approval for clinical investigations.
Key Components of Form MD-23
Form MD-23 is a regulatory document issued by the Central Licensing Authority granting permission to conduct clinical investigations for investigational medical devices. This form outlines the details of the investigational device, the clinical investigation sites, and the conditions under which the investigation is approved, ensuring compliance with the Medical Devices Rules, 2017. Here are the key components of the form:
- Grant of Permission: The sponsor is granted permission to conduct the clinical investigation at specified sites.
- Device and Site Information: Detailed information about the investigational medical device(s) and clinical investigation sites is annexed.
- Conditions for Approval: The permission is subject to compliance with the Medical Devices Rules, 2017.
- Investigational Device Details: Includes the generic name, intended use, and class of the medical device, and other technical details.
- Investigation Site Details: Specifies the name, address, ethics committee details, and the principal investigator’s name for each site involved.
- Digital Signature: The form must be signed digitally by the Central Licensing Authority.
Table 1 : outlines the process for applying for clinical investigation permission:
| Step | Action |
| Step 1 | Apply for a grant of permission to conduct a clinical investigation using Form MD-22. |
| Step 2 | Address the application to the Central Licensing Authority of India and the Central Drugs Standard and Control Organisation (CDSCO). |
| Step 3 | Apply along with the required documents and application fees via the SUGAM portal. |
Best Practises for Successful Approval
To enhance the likelihood of approval, applicants should consider the following best practices:
- Ethics Committee Approval: Before conducting the clinical investigation of investigational medical devices, securing approval from the Ethics Committee is mandatory.
- Biocompatibility Test Reports: Ensure that biocompatibility test reports are included with the application as they are required to proceed with the clinical investigation.
- Clinical Investigation Plan: The submission of the Clinical Investigation Plan is essential for the application process.
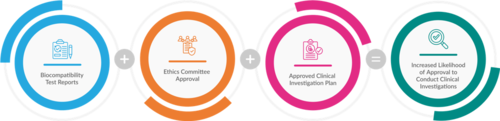
Fig. 2: Strategies to Ensure Successful Approval for Clinical Investigations
To avoid potential issues during the application process, applicants should keep the following in mind:
- The Clinical Investigation Plan must first be approved by a registered Ethics Committee before initiating the study.
- The first participant must be enrolled within one year from the date of permission for the clinical investigation to commence. If the recruitment is not completed within this timeframe, prior approval from the Central Licensing Authority will be required to proceed.
- In case of any injury or death of a subject during the clinical investigation, the applicant is obligated to provide complete medical management and compensation, as per the Medical Device Rules, 2017 (MDR, 2017).
Conclusion
In conclusion, securing approval for clinical investigations is a fundamental step in ensuring the safety and effectiveness of investigational medical devices. The submission of Forms MD-22 and MD-23 certifies adherence to the necessary regulatory and ethical standards during the clinical investigation phase. CliniExperts, with their specialised knowledge in clinical research and regulatory affairs, streamline the application process by ensuring accurate documentation, offering guidance on ethical and scientific aspects, and navigating complex regulations. Their expertise helps minimise delays, reduce errors, and improve the chances of successful approval, adding value all along the clinical investigation process of your medical device.
References:
1. CDSCO Guidelines. [Internet] Central Drugs Standard Control Organisation [cited 2024 December 23]. Available from: https://cdscomdonline.gov.in/NewMedDev/resources/app_srv/NMD/global/pdf/CDSCO-guideline.pdf
2. Permission to conduct a clinical investigation in India. [Internet] CliniExperts [cited 2024 December 23]. Available from: https://cliniexperts.com/india-regulatory-services/medical-device/permission-to-conduct-clinical-investigation-license-in-india/
3. Form MD-22. [Internet] Central Drugs Standard Control Organisation [cited 2024 December 23]. Available from: https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2022/m_device/Medical%20Devices%20Rules,%202017.pdf
4. Medical Devices Frequently Asked Questions. [Internet] Central Drugs Standard Control Organisation [cited 2024 December 23]. Available from: https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf-documents/mdfaq24.pdf
Form Names: MD3, MD5
Permission to Manufacture Class A-B Medical Devices
The licensing authority in India is the Central Standard Control Organisation (CDSCO). The licensing process in a nutshell:
Fill up the exhaustive application Form MD-3 and upload it on the SUGAM portal State licensing authorities issue permission to manufacture Class A and B devices on Form MD-5 after a grueling evaluation process
Regulatory Body Requirement
Manufacturing medical devices requires stringent processes to be completed under the laid down rules and regulations by Central Drug Standard Control Organisation (CDSCO). In addition, applicants who intend to manufacture Class A or Class B medical devices have to go through a procedure by applying for a license to sell or distribute medical devices. The CDSCO ensures quality and safety of medical devices sold in the market in this manner.
Such applicants have to apply to the State Licensing Authority for approval from the Ministry of Health and Family Welfare’s online portal, depending on the applicant’s location. An application has to be submitted in FORM MD-3 along with necessary documents for procuring a license as FORM MD-5.
How to Apply
A manufacturer should apply via the FORM MD-3 to the State Licensing Authority for manufacturing, sales or distribution of medical devices. A list of documents is required to be submitted along with the FORM MD-3, including;
- Step 1: Collect a list of essential documents like Cover letter, Location ownership/ agreement/ tenancy, details of constitution of the firm, test license, IVD`s, quality control data
- Step 2: Apply and submit through Form MD-3 to the State Licensing Authority for manufacturing, sales, or distribution of Medical Devices.
- Step 3: Wait for the review process of the submitted documents and Forms
- Step 4: Allow an inspection of the manufacturing site conducted for Class B Medical Devices
- Step 5: After the review, the State Licensing Authority will grant the manufacturer permission and the manufacturing license in Form MD-5
Recent Posts
Cosmetic Label Compliance India : A Guide to Compliance

Introduction Looking for Cosmetic Label Compliance India? Are you a cosmetic manufacturer or importer navigating the complex world of Indian regulations? Ensuring your product labels comply with the l..
Regulatory Challenges of SaMD: Navigating Global Standards and Compliance

Navigating Software as a Medical Device regulation involves addressing varying global standards, including risk-based classifications...
Lorem ipsum dolor sit amet consectetur adipisicing elit. Maiores, praesentium. Delectus distinctio velit rerum dignissimos aliquid harum, facere, dicta atque unde a totam! Officiis modi non magni quam sapiente numquam voluptatibus fugiat quidem nobis in et eum harum aperiam nostrum quo, minus dignissimos odio, dicta error enim. Culpa, reiciendis facilis.
HAVE A QUERY?
REACH US!Office
New Delhi
Unit No. 324 & 325, City Centre Mall, Plot No. 5, Sector 12, Dwarka, India - 110075
+917672005050
Bengaluru
RMZ Galleria, 1st floor, Ambedkar Colony, Yelahanka, Bengaluru, Karnataka, India – 560064
Call us on
Sales: +91 7672005050
Reception: +91-11-45214546
Timings
9 am to 6 pm (Monday to Friday)


