New Notification on Regulating All Devices – Deciphering the Key Concepts
The Medical Devices Rules (MDR), 2017 came into effect from 1st January, 2018. On February 11, 2020, two major notifications related to the Medical Devices Rules, 2017 were published by the Government of India. The notifications included:
- A new definition of medical devices.
- The Medical Devices (Amendment) Rules, 2020
As per the new regulations, from April 1, 2020 (which is the effective date), all medical devices will be under the scrutiny of quality and safety regulation New Definition of Medical Devices – Looking beyond the 37 notified categories
As of now (until February 11, 2020), only 37 categories of medical devices were regulated or notified as drugs. From April 1, 2020, the medical devices that fall under the following definition will be regulated as “drug” under the Drugs and Cosmetics Act, 1940 (DCA) and MDR:
All devices including an instrument, apparatus, appliance, implant, material or other article, whether used alone or in combination, including a software or an accessory, intended by its manufacturer to be used specially for human beings or animals which does not achieve the primary intended action in or on human body or animals by any pharmacological or immunological or metabolic means, but which may assist in its intended function by such means for one or more of the specific purposes of ―
- Diagnosis, prevention, monitoring, treatment or alleviation of any disease or disorder;
- Diagnosis, monitoring, treatment, alleviation or assistance for, any injury or disability;
- Investigation, replacement or modification or support of the anatomy or of a
- physiological process;
- Supporting or sustaining life;
- Disinfection of medical devices; and
- Control of conception
The Medical Device (Amendment) Rules, 2020
As per the MDR amendment, a new chapter (Chapter IIIA – Registration of certain medical devices) has been introduced for registration of “Newly Notified Medical Devices” by their respective manufacturers and importers. The new rule exempts the 37 categories of already regulated or notified medical devices from the requirement of registration.
Registration Process
As per the amendment, the manufacturers or importers of the “Newly Notified Medical Devices” will be required to register their medical devices with the Central Licensing
Authority through a dedicated online portal established by the Central Drugs Standard Control Organisation. The registration shall be on voluntary basis for a period of eighteen months (1st April 2020 to 30 Sep 2021), from the commencement of this rule, after which (from 01 October 2021), it shall be compulsory.
Below is a snapshot of the timelines for obtaining the registration number
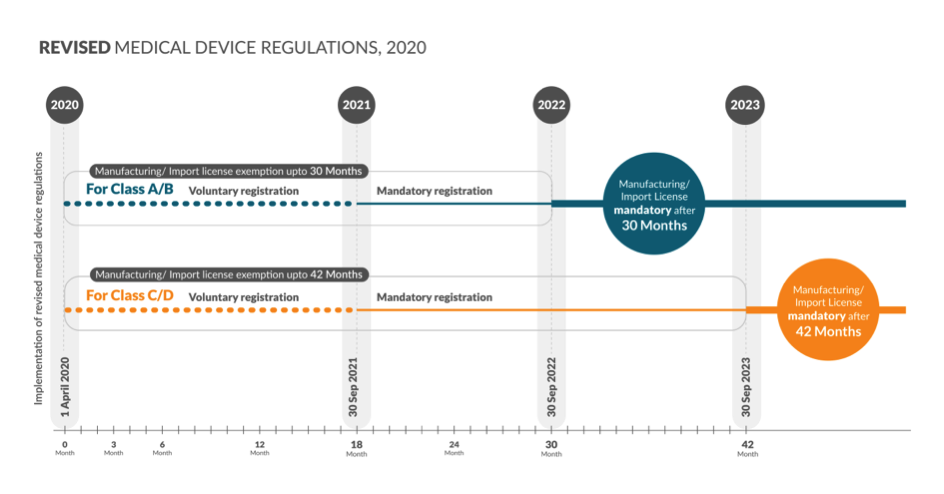
Mandatory registration will start from 30 Sep 2021 for Class A, B, C and D:
Before October 1, 2021, all presently unregulated medical devices will have to be registered by respective importers or manufacturers with the Drugs Controller General of India.
Time frame for Class A and Class B – 30 Months from 1st April 2020:
Before August 11, 2022, the manufacturer/importer of currently unregulated low risk – Class A and low moderate risk – Class B medical devices will have to mandatorily obtain a license and get a registration number.
Time Frame for Class C and Class D devices – 42 Months from 1st April 2020:
Before August 11, 2023, the manufacturer/importer of currently unregulated moderate high risk – Class C and high risk – Class D medical devices will have to mandatorily obtain a license and get a registration number.
The following information needs to be uploaded while registering:
- Name and address of the company or firm or any other entity manufacturing the medical device.
- Name and address of manufacturing site of medical device.
- Specific details of the medical device:
- Generic name
- Model no.
- Intended Use
- Class of Medical device
- Material of Construction
- Dimension (if any)
- Shelf Life
- Sterile or Non Sterile
- Brand Name (if registered under the Trade Marks Act, 1999)
- Certificate of compliance with respect to ISO 13485 standard accredited by National Accreditation Board for Certification Bodies or International Accreditation Forum in respect of such medical device.
- Free sale certificate from country of origin. (Applicable to importers of the new category of Medical Devices)
- Undertaking duly signed by the manufacturer/importer stating that the information furnished by the applicant is true and authentic.
Registration number – Needs to be mentioned on the device label
After all the required information is uploaded to the “Online System for Medical Devices”, a registration number will be generated and the manufacturer/importer will be required to mention the registration number on the label of the medical device.
Safety and Quality checks by the Central Licensing Authority
To ensure quality and safety, the Central Licensing Authority may verify the documents at any point of time and investigate any quality or safety related failure or complaints. The CLA has the right to revoke/suspend the registration if it feels the registrant is not complying with the said quality/safety parameters.
Summary
- From 01 April 2020, all medical devices that meet the medical device definition under notification S.O. 648(E) dated 11.02.2020 will be regulated as drugs within India.
- As per the MDR amendment, a new chapter has been introduced for registration of “Newly Notified Medical Devices” in which the manufacturers or importers of the will be required to register their medical devices with the Central Licensing Authority through a dedicated online portal established by the Central Drugs Standard Control Organisation.
- The registration shall be on voluntary basis for a period of eighteen months (1st April 2020 to 30 Sep 2021), from the commencement of this rule, after which (from 01 October 2021), it shall be compulsory.
- After end of the voluntary registration period, it shall be mandatory to obtain the registration number. The timelines for obtaining the registration number will be 30 months for Class A (low-risk) and Class B (medium-risk) medical devices and 42 months for Class C (low-risk) and Class D (medium-risk) medical devices.
References
- Ministry Of Health and Family Welfare. Notification. GSR102E. Registration of certain medical devices. 11th February, 2020. https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/downloa d_file_division.jsp?num_id=NTU0OQ==
- Ministry Of Health and Family Welfare. Notification. Medical Device Definition.11th February, 2020. Available at: https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/downloa d_file_division.jsp?num_id=NTU0OA==
Recent Posts
Organic Food Labelling In India| Certification, and Import of Organic Food in India

This Article is All About Organic Food Labelling In India and Certification, and Import of Organic Food in India. Explained in Detail About What is Organic Food labelling? Summary Short Description Wi..
Cosmetic Label Compliance India : A Guide to Compliance

Introduction Looking for Cosmetic Label Compliance India? Are you a cosmetic manufacturer or importer navigating the complex world of Indian regulations? Ensuring your product labels comply with the l..
Clinical Investigation Approvals: An Overview of Forms MD-22 and MD-23

Summary Short Description Strict regulatory protocols govern clinical investigations for medical devices. Central to this process are forms MD-22 and MD-23. Form MD-22 is an application to Central Lic..
HAVE A QUERY?
REACH US!Office
New Delhi
Unit No. 324 & 325, City Centre Mall, Plot No. 5, Sector 12, Dwarka, India - 110075
+917672005050
Bengaluru
RMZ Galleria, 1st floor, Ambedkar Colony, Yelahanka, Bengaluru, Karnataka, India – 560064
Call us on
Sales: +91 7672005050
Reception: +91-11-45214546
Timings
9 am to 6 pm (Monday to Friday)


