Test License for Medical Devices in India - Form MD 16 & MD 17

Need a permission to import medical device in India to demonstrate its performance? CliniExperts’ professionals have expertise and assist you in securing a medical devices test license for importers in Form MD 17 by CDSCO.
Permission To Import Test License For Medical Devices (Form MD 16, 17) – Overview

Who Can Apply?
Any person who imports in India can apply for Test License
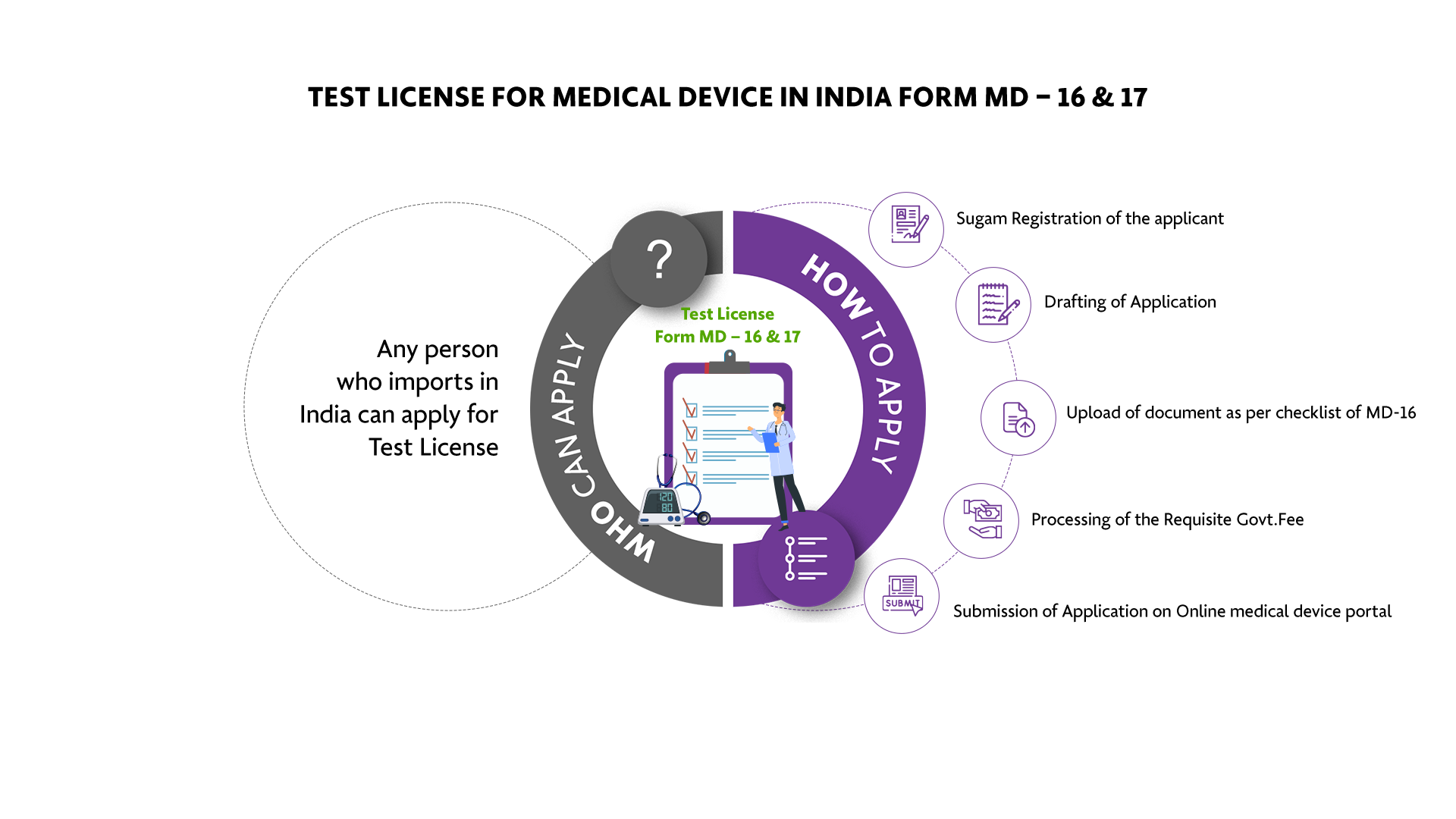
How To Apply?
The Applicant must follow the following process:
-

Sugam Registration of the applicant
-

Drafting of Application
-

Upload of document as per checklist of MD-16
-

Processing of the Requisite Govt. Fee
-

Submission of Application on Online medical device portal

Validity
The test license is valid for three years.

Fee Involved
100 USD per product is the prescribed fee for obtaining a medical device test license in Form MD-17.
Important Documents

- The description of the in vitro diagnostic kit or medical device
- The description must include materials of construction, intended use, design label, and instructions for the use of the medical device.
Timeline to get
MD 17
from CDSCO
30
WORKING DAYSEssential Tips
The applicant looking for permission to import medical devices must ensure these essentials are followed:
- Quantity: The applicant must specify the quantity of medical devices to be imported during the submission.
- Batch Details: The device's batch details, quantity to be utilized, and amount to be retained must be justified during the submission.
- Other Details: The name of the place where testing, evaluation, demonstration, or training will be carried out should be mentioned during the submission.
- Clinical Trial Details: If the test license is required for clinical investigation, the protocol of the clinical trials must be given.
Expert Advise
After obtaining the test license, the individual must keep a record of all date and quantity of imported medical device and the manufacturer's name.
The license holder must keep a record of an invoice or statement that has details of the name and quantity of medical device imported.
The license holder must only use the medical device for the purpose it was imported.
Related Services
Import License for Medical Devices in India
Importer | Regulatory Body: CDSCO
Meet all your Regulatory Compliance needs. CliniExperts' professionals help you plan and streamline regulatory approval processes.
Non-Notified Medical Devices Registration/ Approval in India
Importer | Regulatory Body: CDSCO
CliniExperts acts as an authorized representative to help you in getting the permission to import non-notified medical devices in India. Register your Non-notified Medical Devices in India with CliniExperts' professional assistance
SUGAM Registration- MD/ IVD
Importer | Regulatory Body: CDSCO
The registration process includes several steps to obtain approvals. The process involves registration, undertaking, and uploading the documents, complying with the given rules and regulations. We at CliniExperts, make sure that our expert team creates your registration easy and quick so that you can focus on more important aspects of your product launch...
Frequently Asked Questions
Can the applicant mention multiple sites in a single test license application?
Yes, the applicant can mention multiple sites in a single test license application for the test, evaluation, demonstration, training, or clinical investigation of the medical device.

If the Central licensing authority cancels the license, when can the license holder appeal to the authority?
The license holder can appeal to the Central licensing authority 45 days after cancellation of the license.

Which products are not regulated under MDR 2017?
The following list of products are not regulated under MDR 2017:
- Research Use Only
- Q.C material for accreditation
- Panel for Q.C testing
- Product used for food, water, sterility testing used by various industries for Q.C
- Microbiological culture media
- Stains indicators
- Reagents used for food and water testing.

