Food Regulatory Services
Over the years, we have forged an in-depth understanding of the rules laid down by the FSSAI through careful monitoring of the changing laws and constant professional liaison with apex regulatory bodies of India. Our committed team of FSSAI registration experts is constantly prepared for challenges and changes in order to help you achieve your goal along with our license without any hassles. All matters related to FSSAI license, registration, compliance and import assistance is handled by us at CliniExperts with strategic planning and technical acumen.
Product Compliance as per FSSAI Guidelines
Compliance Check for Formula
Categorization of Products/Ingredients
Label and Claims Validation
Claims Substantiation
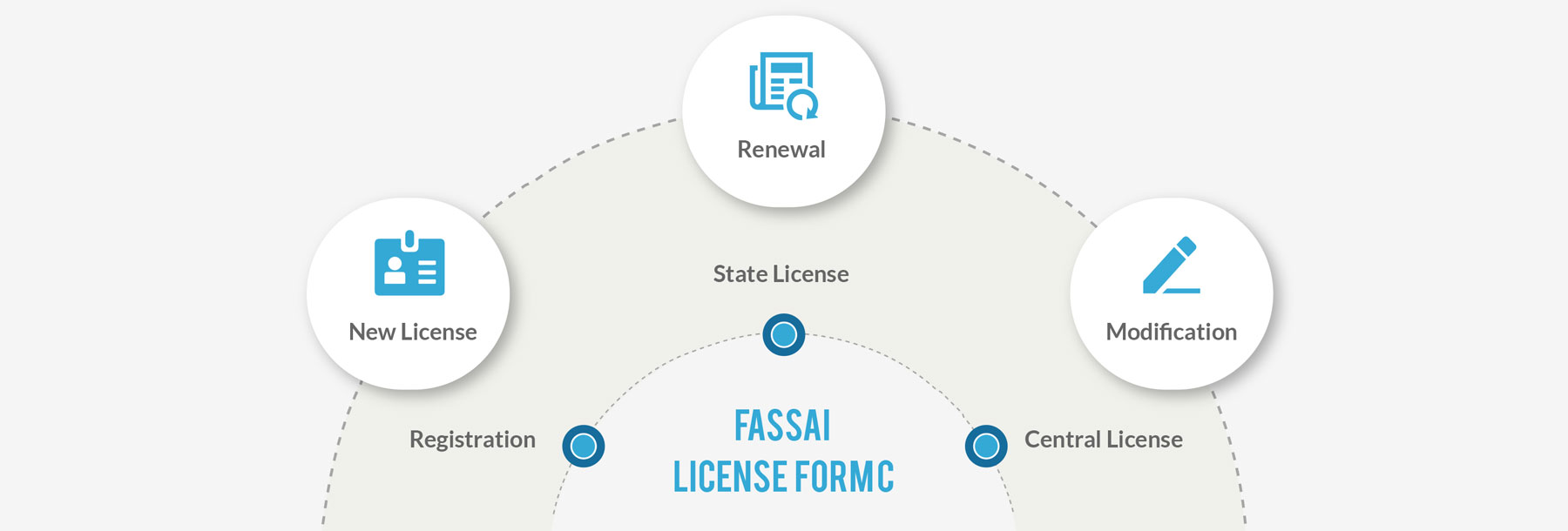
FSSAI License (Form C) – Registration, State and Central License
Registration
State License
Central License
Non – Specified Product/ Ingredient Approval
Import Assistance
IEC Number
FSSAI Importers License
Product Compliance
Non-Specified Product/ Ingredient Approval
Sanitary Import Permit for Food Products and Ingredients
Product Compliance as Per FSSAI GUIDELINES
Getting an FSSAI license is not enough. One must comply with the Food Safety and Standards Regulations. There are several guidelines and regulations under FSSAI which keeps on updating. This makes it difficult for the Food Business operators in India to keep themselves updated. We provide authorized support to our clients for the FSSAI compliance of their food products and facilities in India. Being a consultant, we offer the complete guidance and solutions in following systems:
Compliance check
Compliance check for formulas
Analysis of product formula and/or ingredients are required to ensure the use of approved ingredients and additives within their permissible limits as per the Food Category laid down by FSSAI in respective regulations and standards. We also ensure the RDA compliance of nutrients as per the product’s category and claims.
Label and Claims Validation
Label and Claims Validation
There are numerous regulations for food packaging and labelling to be followed in India as set by FSSAI. All the food business operators in India need to comply with the minutest details of these regulations. We assist our clients in ensuring compliance of the label as per the Packaging and Labeling Regulations by FSSAI.
The claims on products and ingredients for labeling and advertisement purpose are also restricted by a set of regulations with certain provisions. Our team provides guidance and support for the validation of claims as per the required standards.
Claims Substantiation
Claims Substantiation
Understanding the need of the product, we assist our clients in the substantiation of required claims for labeling and advertisement purpose. We also help them conduct trials on their specific products including functional foods for the required purposes.
Categorization of Products/Ingredients
Categorization of Products/Ingredients
Based on the formula analysis and its intended use, the product has to be categorized as per the Food Categorization System and Standard categories mentioned under the Food Safety and Standards Regulations. We enable the food business operators to categorize their products in the appropriate category as per the intended use, which enables them to make the right claims.
FSSAI LICENSE (FORM C) – Registration, State
and Central License
All Food Business Operators in India are mandatorily required to have an FSSAI issued license for their premises as per the kind of activities they perform. We guide our clients through the entire journey of obtaining FSSAI License or Registration.
Through our extensive network and professional liaison with the Regulatory Authorities, we support FBOs such as manufacturers, importers, re-packers, re-labelers, retailers, caterers, restaurateur, hotelier, wholesaler, storage units, food transporters, canteens, food processing units and any other food business operators in food chain get a license depending upon the activity, size and scale of business.
Our Services also include Modification, Renewal and obtaining Duplicate License.
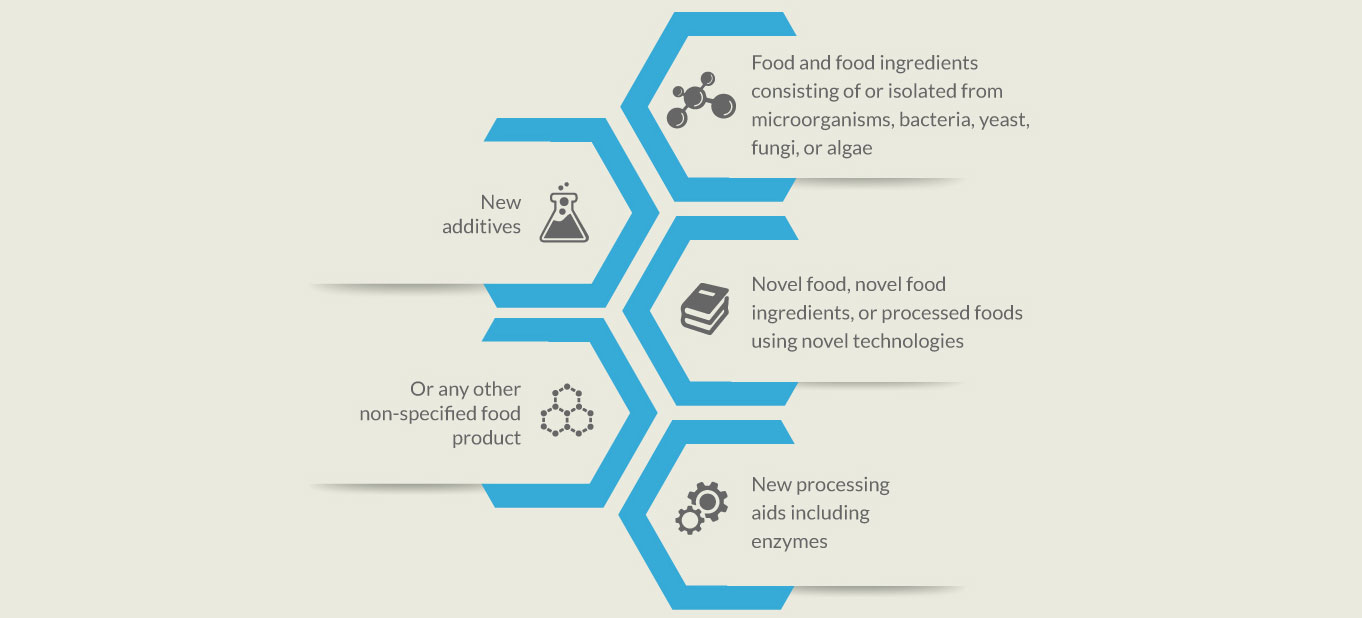
Non–Specified Product / Ingredient Approval
FBOs that manufacture or import the products falling outside the purview of established Food standards and categorization system, are required to file an application for the Non-Specified Food Product or Ingredient Approval with FSSAI in the applicable category:
We at CliniExperts extend our support throughout the process of preparing and filing of good quality applications to FSSAI Non-Specified Food Approval department. We carry out a checklist based evaluation of all the documents before submission, in order to ensure that all the requirements have been met ensuring fast pace approval. Our dedicated team constantly keeps a track of application to ensure that the product approval process is carried out smoothly. We have a proven track record of supporting numerous prestigious brands achieve fast product approval from FSSAI.
Import Assistance
We provide complete regulatory solutions for Food Business Operators trying to venture in India and the Indian importers of food products for all the compliance related activities as per the regulatory requirements, helping them understand and overcome the regulatory obstacles.
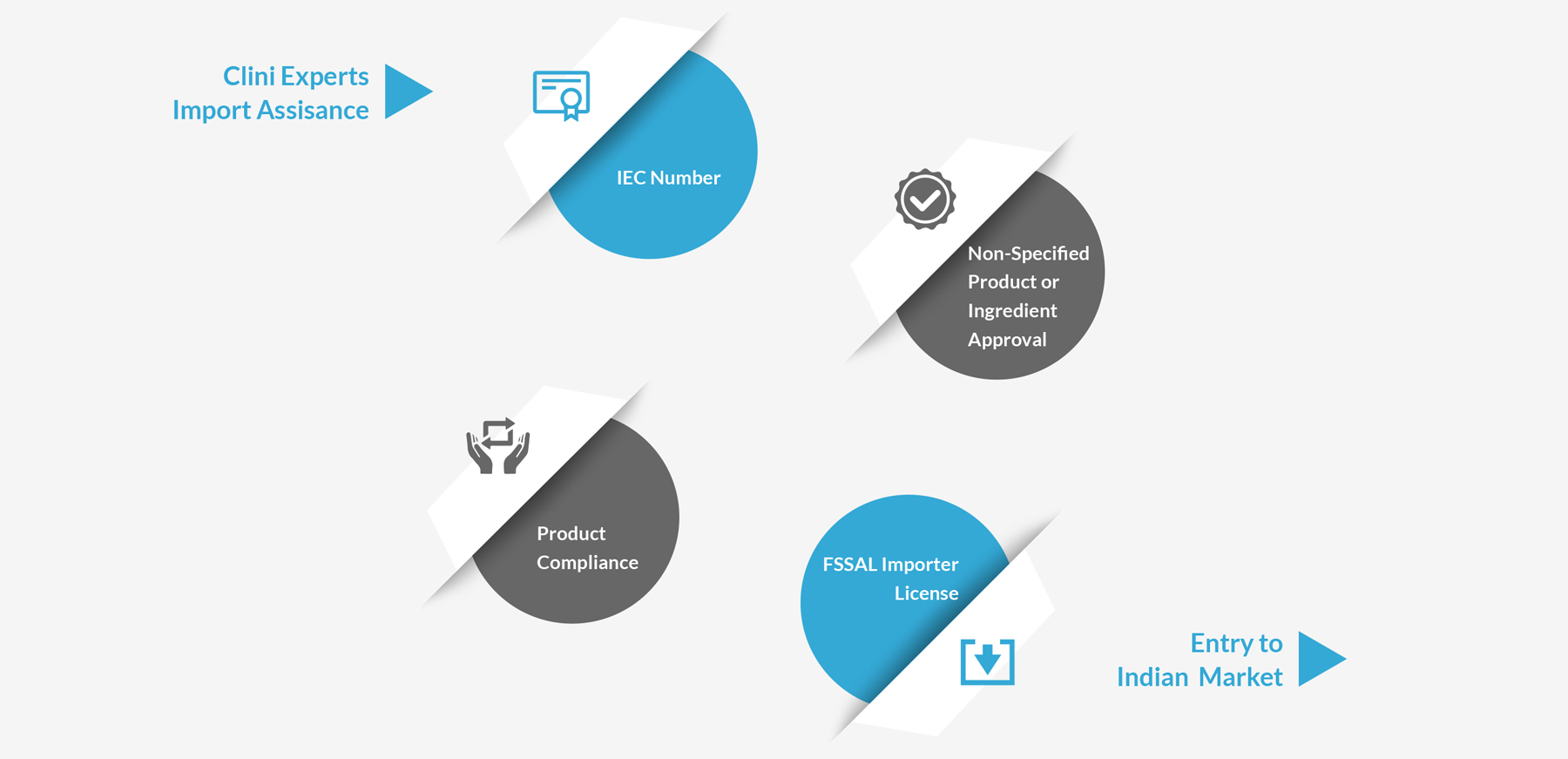
Entry to Indian Market

IEC
Number

FSSAI Importers
License

Product Compliance as per FSSAI Guidelines & Non-specified Product Approvals

Sanitary
Import Permit
OUR Credentials
Obtained
Compliance as
per FSSAI
OUR Clientele




























Related Services
Wholesale Drug License in India
Importer | Regulatory Body: SLA
CliniExperts offer strategic planning services for Wholesale Drug License to start with your pharmaceutical business and sell drugs at distributor level. Our hand in glove approach ensures that at no point you find yourself battling with any process by yourself.
Clarification Letter / No Objection Certificate for Medical Devices in India
Importer | Regulatory Body: CDSCO
Unclear about the regulatory status of Medical Devices in India. Let CliniExperts’ professionals assist you for getting a Clarification Letter / No Objection Certificate (NOC) for Medical Devices from Central Drugs Standard Control Organisation.
Test License for Medical Devices in India
Importer | Regulatory Body: CDSCO
Need a permission to import medical device in India to demonstrate its performance? CliniExperts’ professionals have expertise and assist you in securing a medical devices test license for importers in Form MD 17 by CDSCO.
Non-Notified Medical Devices Registration/ Approval in India
Importer | Regulatory Body: CDSCO
CliniExperts acts as an authorized representative to help you in getting the permission to import non-notified medical devices in India. Register your Non-notified Medical Devices in India with CliniExperts professional assistance
Authorized Agent For In-Vitro Diagnostic Kits
Importer | Regulatory Body: CDCSO
Importing an in-vitro diagnostic kit and selling it across the country can be overwhelming if you do not have any local establishments in India. With a well-established presence in India, CliniExperts can help you comply with CDSCO requirements and start selling your device in this emerging market. CliniExperts hold a drug wholesale license in Form 20-B and 21-B and can be your in-country representative and IVD importing a hassle-free process.
Permission to import or manufacture new medical device in India
Importer | Regulatory Body: CDSCO
Get experts assistance to avail Permission to import or manufacture medical device which does not have its predicate device in India -As per MDR 2017

Regulation/Guidelines
Update on Food Safety and Standards (Labelling and Display) Regulations Draft, 2018
The Food Safety and Standards Authority of India on April 11, 2018 has published the Draft for Food Safety and Standards (Labelling and Display) Regulations, 2018. These regulations propose to prescribe the labelling requirements of pre-packaged foods and display of essential information on premises where food is manufactured, processed, served […]
Read MoreInsight
FSSAI Draft Norms for E-Commerce FBO’s
To ensure safety FSSAI has come up with draft norms for e-commerce FBO's. It is now mandatory to obtain licenses for all online Food Businesses and supply chain
Read MoreInsight
Regulatory Guidelines for Proprietary Food in India
Food Safety and Standards Authority of India (FSSAI) is actively working in rendering safety and nutritiously balanced food to the people of the country. Recently, the new guidelines for proprietary food in India came into force from August 22, 2016.
Read More
Regulation/Guidelines
Expected Changes in FSSAI Labeling Regulations and Gazetted Alcoholic Beverage Regulations
The Food Safety and Standards Authority of India on April 5, 2018, published a gazette notification of Food Safety and Standards (Alcoholic Beverages) Regulations 2018 and on April 11, 2018, it published the Draft Food Safety and Standards (Labelling and Display) Regulations, 2018.
Read MoreNews
FBO must adhere to FSSAI guidelines, published on website of regulator
Health minister Dr Harsh Vardhan stated that food business operators (FBO) have to adhere to the advisories and guidelines issued by FSSAI since 2011, the year the Food Safety and Standards Regulations (FSSR) were implemented
Read MoreRegulation/Guidelines
Comparison of Initial Draft and Final Gazette Regulations for Alcoholic Beverages
FSSAI has framed regulations specifying the standards for the production and packaging of alcoholic beverages, the draft of which was released in September 2016. With Amendments, these regulations i.e. Food Safety and Standards (Alcoholic Beverages Standards) Regulations, 2018, specify the standards for manufacturing Alcoholic beverages and their packaging which include: […]
Read MoreNews
Self-Assessment/Audit of Unit for GMP/GLP Compliance
The Indian government has brought about some major changes with regards to the rules and regulations governing the manufacturing to enhance the quality of products used in the healthcare industry. As India being a major market for the healthcare-related products and its services, these modifications to the existing regulations are […]
Read MoreInsight
FSSAI Regulations for Caffeinated Beverages
FSSAI has added standards for caffeinated beverages and use of blue tint in the plastic bottles related to beverages non-alcoholic carbonated food products
Read More