Permission to conduct Clinical Performance Evaluation in India - MD-24 & MD-25

CliniExperts has an experienced team who guides with all the paperwork for regulatory procedures. They also help in getting permission to conduct a Clinical Performance Evaluation for new IVDs in form MD-25.
Permission to conduct Clinical Performance Evaluation – Overview
The Central Licensing Authority, CDSCO, is the regulatory body associated with this service. This service aims to obtain Permission to conduct a Clinical Performance Evaluation of all new IVDs.
A manufacturer or an importer who intends to conduct a Clinical Performance Evaluation of a new IVD can apply through this service.
A manufacturer or an importer who intends to conduct a Clinical Performance Evaluation of a new In-vitro diagnostic can apply through this service.
- The applicant needs to make an application to the Central Licensing Authority.
- The application can be filled in FORM MD-24 through Medical Device portal of the Ministry of Health and Family Welfare.
- The Permission to conduct a Clinical Performance Evaluation will be obtained in FORM MD-25.
- The approval is a government process and could take approximately 1 to 2 months.
Who Can Apply?
Any manufacturer or importer who wishes to conduct a Clinical Performance Evaluation of new IVD can make an application to the CLA.
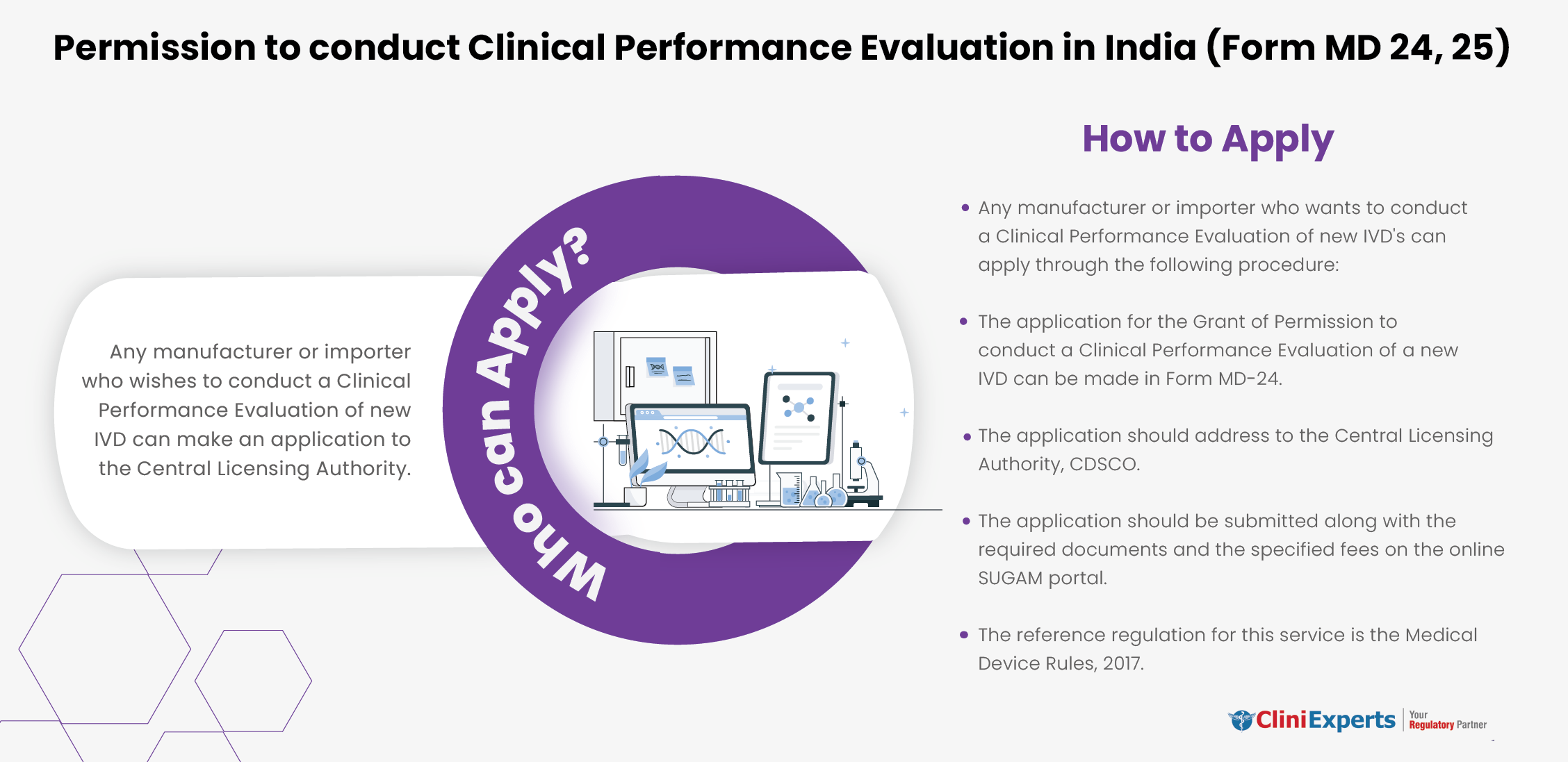
How To Apply?
The Applicant must follow the following process:
-

Step 1: The application for the Grant of Permission to conduct a Clinical Performance Evaluation of anew IVD can be made in Form MD-24.
-

Step 2: The application should address to the Central Licensing Authority, CDSCO.
-

Step 3: The application should be submitted along with the required documents and the specified fees on the online SUGAM portal.
-

Step 4: The reference regulation for this service is the Medical Device Rules, 2017.

Validity
The license validity for this service is not applicable.

Fee Involved
The Government charges fees of 25000 INR for obtaining permission to carry out a Clinical Performance Evaluation of a new IVD.Important Documents

While making an online application for obtaining a Grant license for selling products, the applicant must upload the following documents:
- Undertaking by investigators
- Approval from the Ethics Committee
- Clinical Performance Evaluation plan
- In-house Performance Evaluation data (used to establish sensitivity, specificity, stability, reproducibility and repeatability)
- Product Description
- CRF (Consent report form)
Timeline to get
MD-25
from Central Drugs Standard Control Organisation
1 to 2
MONTHSEssential Tips
CliniExperts has some essential tips that might benefit their clients.
- The applicant must have an undertaking from investigators.
- The evaluation plan should outline the purpose, technical or medical grounds and scope of the evaluation.
- The applicant must possess approval from an Ethics Committee registered with the CLA.
- A few problems can be faced if the following points are left unchecked during the application process:
- Before enrolling the first participant, applicant must register with Clinical Trial Registry.
- The evaluation plan must be initiated only after the registered Ethics Committee approves.
- The evaluation should be conducted as per the guidelines of Good Clinical Practices. It must also adhere to the approved Clinical Performance Evaluation plan.
Expert Advise
CliniExperts provides sure advice to their clients-
The applicant must have approval from the Ethics committee.
The Clinical Performance Evaluation needs to be initiated within one year from the date of grant of Permission. If failed, prior permission from the CLA will be required to start such Clinical Performance Evaluation.
During the evaluation, the source and quantity of samples need to be known.
Why Choose CliniExperts?
By partnering with CliniExperts, manufacturers benefit from a streamlined, expert-led approach that minimizes the complexity of regulatory compliance, enhances the accuracy of your application, and accelerates your entry into the Indian market.

Expert Guidance
CliniExperts assist with the registration process with regulatory bodies and ensure compliance with all regulatory requirements, including the timely submission of necessary documents.

Thorough Document Review
CliniExperts conduct a meticulous review of all documents, perform a GAP Analysis to identify and address any deficiencies, and communicate any issues promptly to ensure a smooth application process.
Final Review and Fee Management
CliniExperts oversee the final review of your application and coordinate the payment of government fees, ensuring that all financial aspects are handled efficiently.

Timely Follow up
CliniExperts will maintain timely follow-ups with the relevant Government bodies to keep everything on track.
Regulatory Compliance
CliniExperts ensure that all submissions are in strict adherence to the regulatory guidelines
Related Services
SUGAM Registration- MD/ IVD
Importer | Regulatory Body: CDSCO
The registration process includes several steps to obtain approvals. The process involves registration, undertaking, and uploading the documents, complying with the given rules and regulations. We at CliniExperts, make sure that our expert team creates your registration easy and quick so that you can focus on more important aspects of your product launch...
Test license to manufacture In Vitro Diagnostics in India – MD 12 & MD 13
Manufacturers | Regulatory Body: CDSCO
Registration for in-vitro diagnostic medical devices for manufacturing can be time-consuming and tedious; we at CliniExperts will take care of all the registration procedures while you focus energies on planning your product launch. From the application process forms to undertaking a license test, we assist in every registration aspect to help you smoothly start the manufacturing process.
Import License For In-Vitro Diagnostic Kits in India – Form MD14 & MD15
Importers | Regulatory Body: CDSCO
Get Your Import License for In-Vitro Diagnostic Kits (IVD) and Medical Devices with CliniExperts. Hassle-free assistance in providing Import License of Form MD 15.
Frequently Asked Questions
What is a new In-vitro Diagnostics (IVD's)?
A new in-vitro diagnostic is any Medical Device mentioned in sub-clause (A) of clause (zb), which is intended to be used for in vitro diagnostics. The device may not be approved for manufacture, sale, or import by the Central Licensing Authority. It is only being tested to establish its performance for relevant analyse or other parameters, including the required details of technology and procedure.

Is clinical performance evaluation required for grant of Permission to manufacture or import a new IVD of Class A?
No. The Clinical Performance Evaluation may not be necessary; if otherwise, the Central Licensing Authority decides it is necessary depending on the nature of the IVD.

Is CDSCO capable of cancelling or suspending the grant of Permission?
The Central Licensing Authority, CDSCO, may suspend or cancel the grant of Permission if the manufacturer or the importer fails to comply with any of the conditions of the Permission. In addition, CDSCO may partly or entirely cancel the Permission or suspend it for a particular period.

If the approval or marketing authorisation is issued by the competent Authorities in Canada, Japan, E.U., U.K., Australia, and the USA, will it be considered for exemption of Clinical Performance Evaluation of New IVD's (Class B, C and D) in India.
No. The Clinical Performance Evaluation (CPE)will not be exempted. The CPE should be conducted in India to approve new IVDs, irrespective of their regulatory status in other countries.

What are the minimum criteria for evaluation of IVD Kits or reagents intended for Chikungunya, Typhoid, Malaria, T.B., Dengue, Cancer, Syphilis and other Class B and C IVD kits?
The minimum performance criteria of IVDs like clinical specificity, sensitivity, repeatability, accuracy, reproducibility, linearity, variance, etc., should follow the manufacturer's claim in the product insert, IFU and COA.

