Test License to Import In-vitro Diagnostics in India in India - MD 16 & MD 17

Do you need permission to import in-vitro diagnostic kits to India to demonstrate its performance? CliniExperts’ professionals have the expertise and assist you in securing in-vitro diagnostic kits test license for importer.
Test License to Import In-vitro Diagnostics in India – Overview
The Central Drugs Standard Control Organization (CDSCO) has issued a notice. The notice states that a small number of medical devices might be imported into India. The devices belonging to Class A, Class B, Class C, or Class D may be imported in small quantities to India based on Test License in Form MD-17. These devices may be imported for specific purposes. These purposes include clinical investigations, testing, evaluations, demonstrations, or training.
To obtain a Test license in Form MD-17, one must apply. This application is for importing the mentioned devices. It is required for testing, evaluation, demonstration, or training purposes. The application for the import license should be made to the Central Licensing Authority. It must be submitted in Form MD-16. This application process must be completed through an identified online portal of the Ministry of Health and Family Welfare.

Who Can Apply?
The Test License can be applied for by any person who imports in India.
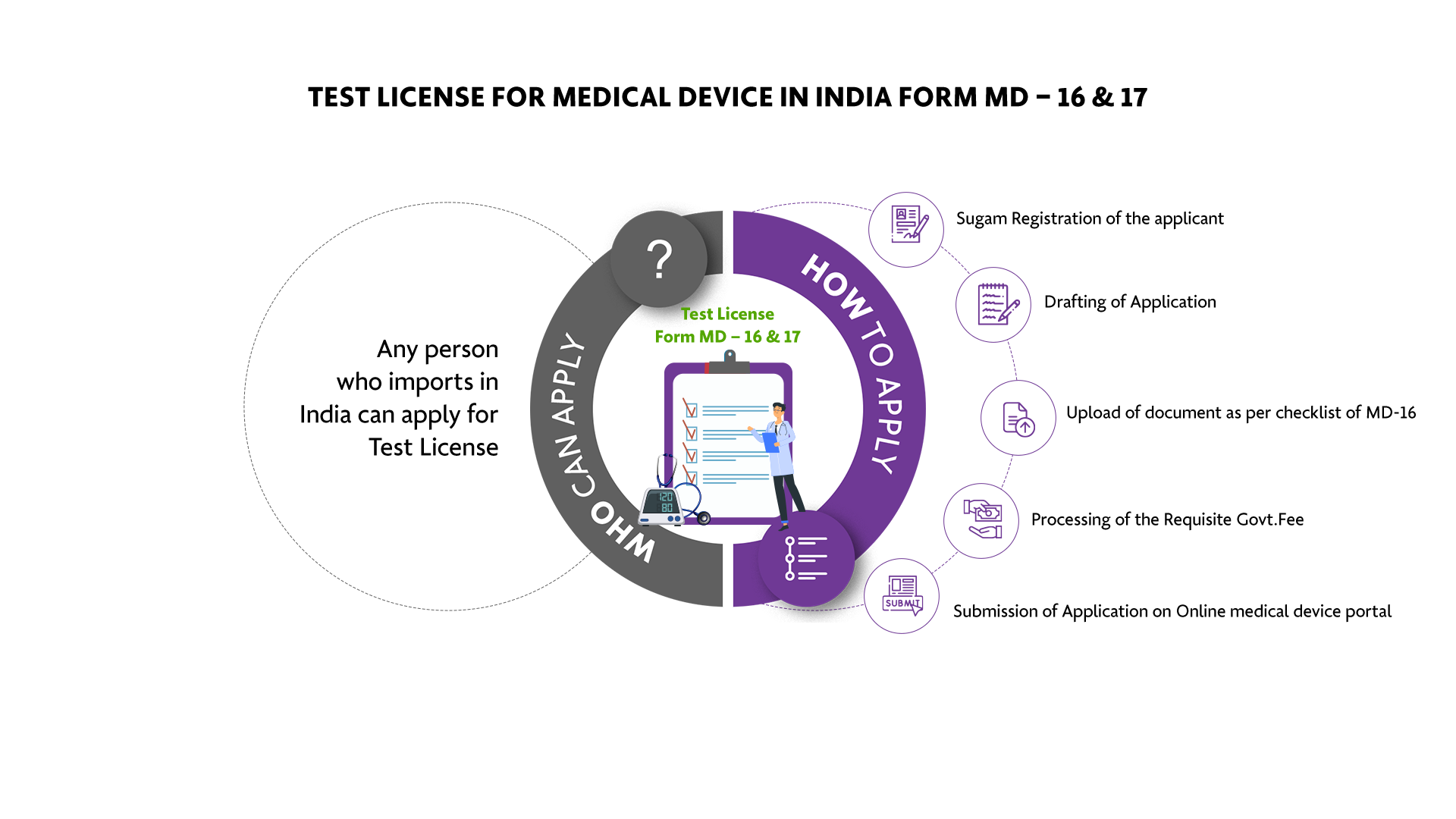
How To Apply?
The Applicant must follow the following process:
-

The professionals at CliniExperts have the expertise and can assist the applicant in securing a test license for importing a medical device in Form MD 17 by CDSCO.

Validity
The test license will be valid for three years.

Fee Involved
The Government will charge a fee of 100 USD for the license.Important Documents

For obtaining a Test License in the Form MD-17, the following major documents will be required:
- A brief description of the in vitro diagnostic kit
- The intended use
- Material of construction
- Design – Label and IFU
Timeline to get
MD 17
from Central Drugs Standard Control Organisation
5 to 7
WORKING DAYSEssential Tips
While applying for the license to import medical devices, the applicant must follow the points stated below:
- The quantity to be imported must be clearly stated and justified in the application to be submitted.
- The justification for the quantity to be imported must be prepared adequately. It should include batch details, quantity to be utilized, and quantity to be retained.
- The name of the place for testing, evaluation, demonstration, training, or clinical investigation is a requirement. It must be finalized and included during submission preparation.
- The name of the place for testing, evaluation, demonstration, training, or clinical investigation is a requirement and must be finalized and included during submission preparation.
Expert Advise
A record of the activities undertaken should be maintained by the test license holder. The records must include the manufacturer's name, quantity import and the date of import.
An invoice or statement showing the medical device's name and quantity should accompany the medical device's consignment.
The Test license should be used only for the purpose it has been obtained.
Related Services
SUGAM Registration- MD/ IVD
Importer | Regulatory Body: CDSCO
The registration process includes several steps to obtain approvals. The process involves registration, undertaking, and uploading the documents, complying with the given rules and regulations. We at CliniExperts, make sure that our expert team creates your registration easy and quick so that you can focus on more important aspects of your product launch...
Test license to manufacture In Vitro Diagnostics in India – MD 12 & MD 13
Manufacturers | Regulatory Body: CDSCO
Registration for in-vitro diagnostic medical devices for manufacturing can be time-consuming and tedious; we at CliniExperts will take care of all the registration procedures while you focus energies on planning your product launch. From the application process forms to undertaking a license test, we assist in every registration aspect to help you smoothly start the manufacturing process.
Import License For In-Vitro Diagnostic Kits in India – Form MD14 & MD15
Importers | Regulatory Body: CDSCO
Get Your Import License for In-Vitro Diagnostic Kits (IVD) and Medical Devices with CliniExperts. Hassle-free assistance in providing Import License of Form MD 15.
Frequently Asked Questions
In a "single” test license application for clinical investigation, testing, evaluation, demonstration, and training, is it possible to mention multiple sites?
Yes

Are the products not intended to be used in humans or animals for the diagnosis of any disease or disorder such as RUO- Research Use Only, Q.C material for accreditation, panel for Q.C testing & product used for food, water, sterility testing used by various industry for Q.C, etc. regulated under MDR, 2017?
No, such products are not regulated under MDR 2017.

When can the licensee whose license has been cancelled by the Central licensing authority appeal to the authority?
The licensee can appeal within 45 days from the date of the order.

Are the products which are not intended to be used in humans or animals for diagnosis of any disease or disorder, such as microbiological culture media, stains indicators and reagents used for food and water testing, be regulated under MDR, 2017?
No, such products are not regulated under MDR 2017.

How can a medical device that is required to be taken to a place other than that mentioned in the test license be relocated?
If such a situation arises, the test license holder must inform the Central Licensing Authority in writing before doing so.

