Endorsement of Predicate In Vitro Diagnostics in Loan License in India - MD 8 & MD 10

Unlock manufacturing excellence with CliniExperts! Secure your MD-10 Loan License for Class C/D Medical Devices effortlessly. With 10+ years of regulatory compliance experience, our expert team ensures swift approvals. Trust our industry network for efficient solutions. Elevate your business today! Contact us for streamlined regulatory success.
Endorsement of Predicate In Vitro Diagnostics in Loan License – Overview

Who Can Apply?
The application is open to any manufacturer of Medical Devices in India.
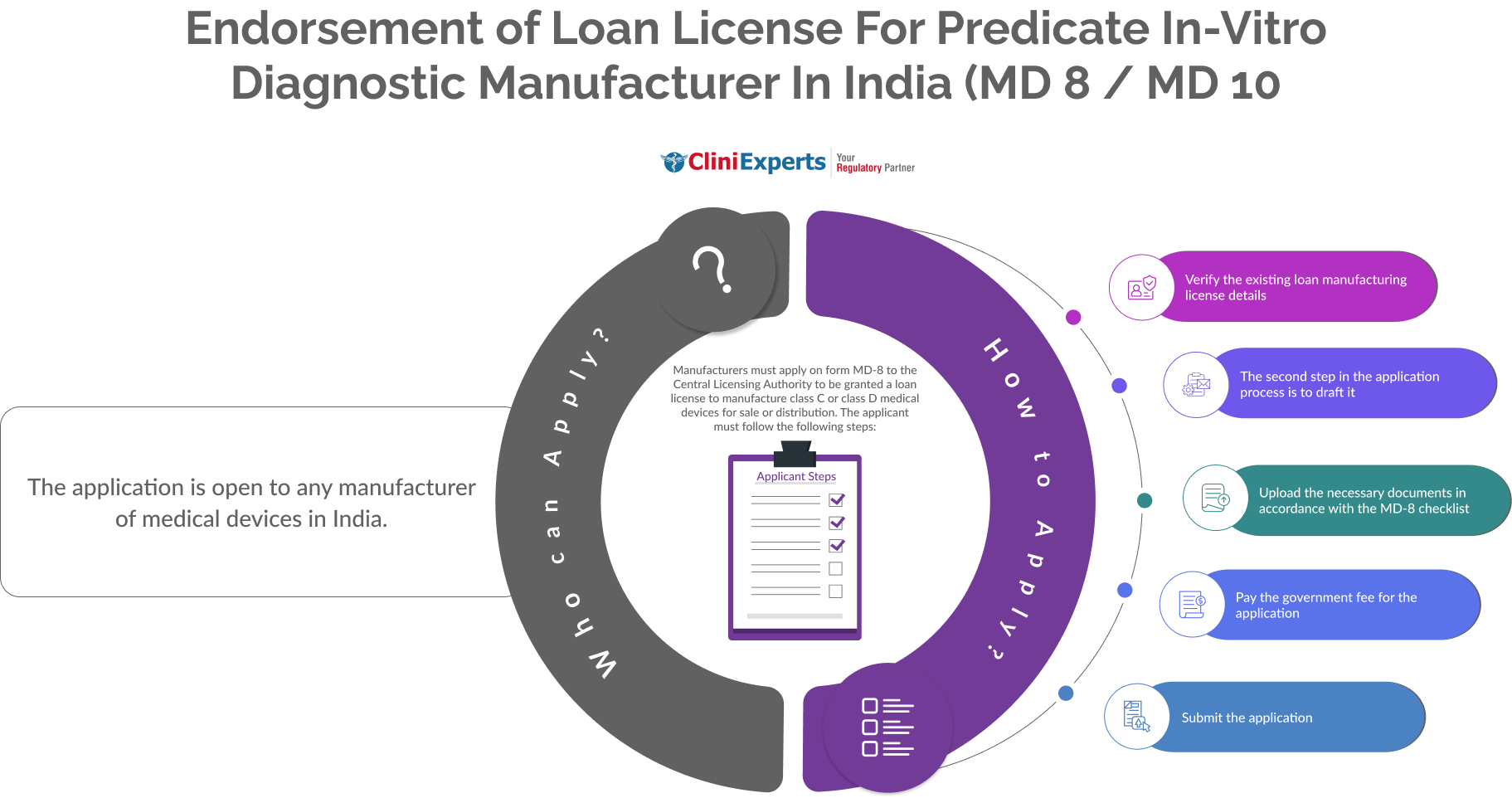
How To Apply?
The Applicant must follow the following process:
-

Verify the existing loan manufacturing license details.
-

The second step in the application process is to draft it.
-

Verify the existing loan manufacturing license details.
-

Upload the necessary documents in accordance with the MD-8 checklist.
-

Pay the government fee for the application.
-

Submit the application.

Validity
A Form MD-10 license stays valid forever if the license retention fee is paid within five years from its issue date, as per the Second Schedule. However, the license can be suspended or canceled by the Central Licensing Authority.
Fee Involved
There is a government fee of Rs. 1000 for the loan license application process for each distinct medical device of class C or class D.Important Documents

- QMS documents
- Device Master File
Timeline to get
MD 10
from Central Drugs Standard Control Organisation
4-6
MonthsEssential Tips
- The applicant's manufacturing facility must meet the Quality Management System (QMS) requirements. They are outlined in the Fifth Schedule.
- Device master files and site master files must be ready in accordance with MDR 2017 format.
- Failure of product technical documentation to comply with MDR 2017.
- The absence of a license from the principal manufacturer as per MDR 2017.
Expert Advise
According to our experts, the manufacturers must generate the quality control data using a valid test license at the time of application submission.
Related Services
Test license to import In Vitro Diagnostics (Form MD 16, 17)
Importers | Regulatory Body: CDSCO
Do you need permission to import in-vitro diagnostic kits to India to demonstrate its performance? CliniExperts’ professionals have the expertise and assist you in securing in-vitro diagnostic kits test license for importer.
Permission to conduct Clinical Performance Evaluation (Form MD 24, 25)
Manufacturer / Importer | Regulatory Body: CDSCO
CliniExperts is backed by a well-informed and experienced team who guides through all the documentation and paperwork required for the regulatory processes.
Permission to Manufacture Class C & D In- Vitro Diagnostics (Form MD 7, 9)
Manufacturer | Regulatory Body: CDSCO
At CliniExperts, our team of experts is thoroughly updated with the latest requirements of the various authorities, enabling complete and meticulous documentation, timely application and quick approvals/licenses.
Permission to Manufacture Class A & B In Vitro Diagnostics (Form MD 3, 5)
Manufacturer | Regulatory Body: CDSCO
The application for manufacturing, sale or distribution of any medical device includes a tedious list of processes and forms CliniExperts team consists of experts working in the same domain with practical experience and knowledge of the governing rules.
Frequently Asked Questions
When can one expect an inspection by CDSCO for the manufacturing site of class C and D Medical Devices?
The applicants can expect the inspection by CDSCO within sixty days from the date of submission of the application.

How many members of the audit team are prescribed in MDR 2017?

If a loan facility is a licensed manufacturing plant, will there be a re-inspection in this case?
If an IVD manufacturing facility already has a license to produce the Medical Device for sale or distribution, no inspection of the facility is necessary to award a loan license to manufacture the device.

